Methane biotransformation in the ocean and its effects on climate change: A review
Mingyang NIU1,2, Wenyue LIANG1,2 & Fengping WANG1,2*
1 State Key Laboratory of Microbial Metabolism, School of Life Sciences and Biotechnology, Shanghai Jiao Tong University,
Shanghai 200240, China; 2 State Key Laboratory of Ocean Engineering, School of Naval Architecture, Ocean and Civil Engineering, Shanghai Jiao Tong University,
Shanghai 200240, China
Received November 8, 2017; revised June 19, 2018; accepted November 7, 2018; published online November 20, 2018
Abstract
Methane is a potent greenhouse gas. Continental margins contain large reservoirs of methane as solid gas hydrate and the dissolved and gaseous forms of methane. Submarine methane seeps along the global continental margins, including the coastal seas, have been estimated to contribute 0.01 to 0.05 Gt of carbon to the atmosphere annually, accounting for between 1% and 5% of the global methane emissions to the atmosphere. Much of this methane is exhausted via microbial anaerobic methane oxidation. Methane biotransformation in the ocean has effects on global climate change. This review mainly introduces the mechanisms of methanogenesis and methane oxidation and describes new findings that will provide information that will improve the understanding of the balance in terms of the generation, migration and consumption of methane in marine environments. Moreover, this review provides new insights into methane biogeochemical cycles and the effects of marine methane budgets on global climate.
Keywords Methane production, Methane oxidization, Marine, Sediment, Climate
Citation: Niu M, Liang W, Wang F. 2018. Methane biotransformation in the ocean and its effects on climate change: A review. Science China Earth Sciences,
61: 1697–1713, https://doi.org/10.1007/s11430-017-9299-4
1. Introduction
The marine environment is the largest ecosystem on Earth, covering approximately 71% of Earth's surface and accounting for 97% of the total volume of water on Earth (Orcutt et al., 2011). This large ecosystem is composed of a water column, sediment and ocean crust ecosystems. Sediment is a very important component of the marine ecosystem, connecting the water column and ocean crust (Schrenk et al., 2010). Marine sediment mainly consists of particles, including terrestrial soil derived from rivers, biological debris or organic matter, and particles that are produced by animals inhabiting marine water (Burdige, 2007). Sediment is distributed along the continental margin (including the continental shelf and slope) and at convergent margins above subduction zones. Thus far, the thickest sediment is more than 10 km in some regions of the ocean (Divins, 2003). Deep marine sediment hosts unique geological features in the forms of mud volcanoes, gas seeps, and gas hydrates (Figure 1), which support suitable habitats for various kinds of living creatures, especially microbial organisms (Orcutt et al., 2011).
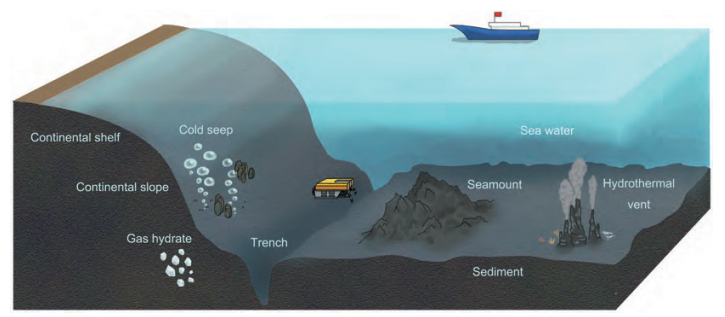
Figure 1 Different geological features and ecosystems of seabed (including cold seep, trench, seamount and hydrothermal vent).
The subseafloor sediment throughout the world contains a large number of microbes and has been estimated to contain 2.9x1029 cells (corresponding to 4.1 petagram (Pg) C and ?0.6% of Earth's total living biomass) (Kallmeyer et al., 2012). Cell counts from these environments generally show little variation between sites and decrease logarithmically earth.scichina.com link.springer.comwith sediment depth. Cell abundance is correlated with sedimentary rate and distance to the coast (Kallmeyer et al., 2012). These cells obtain energy and material from organic matter in sediments.
Microbes (including eukaryotes and prokaryotes) inhabit the surface layer and can oxidize organic matter with oxygen as an electron acceptor to produce carbon dioxide. Compared to sulfate, oxygen is limited in sediment, because it can be easily utilized by microbes. Because oxygen can be easily exhausted in shallow sediment, deep sediment is an anaerobic environment in which nitrate, iron and manganese can act as microbial electron acceptors instead of oxygen (D'Hondt et al., 2002). In anaerobic sediment, complex molecules can be primarily hydrolyzed into oligomers or monomers by extracellular hydrolase secreted by microbes (Weiland, 2010). This step is followed by fermentation, which involves the degradation of these substrates to reduced organic compounds such as short chain fatty acids and alcohols (Na et al., 2015; Ozuolmez et al., 2015). In sulfate- rich sediments, sulfate-reducing bacteria (SRB) can use the products of primary fermentation and oxidize them to CO2 (Muyzer and Stams, 2008). However, in sulfate-depleted methanogenic sediments, short-chain fatty acids and alcohols are converted by secondary fermenters to acetate, formate, H2 and CO2, which are subsequently utilized by methanogenic archaea (MA) to produce CH4 (McInerney et al., 2008; Figure 2). Methane is the final product of anaerobic organic matter degradation and can form crystal clathrate- gas hydrate, which is steadily preserved in sediments when the concentration of methane is saturated at high pressure and low temperatures (temperature below 4 degrees and pressure higher than 60 bar). Methane diffuses into the pores of sediment with increasing concentrations, and is anaerobically oxidized by anaerobic methanotrophs (ANMEs) coupled with SRB to form CO2 in sulfate-rich sediment. A portion of this CO2 forms authigenic carbonate with calcium and magnesium from the surrounding sediment. Other portions can be synthesized to form primary substrates by autotrophic chemosynthesis microbes and recycled via the carbon cycle (Lever, 2012; Figure 2).
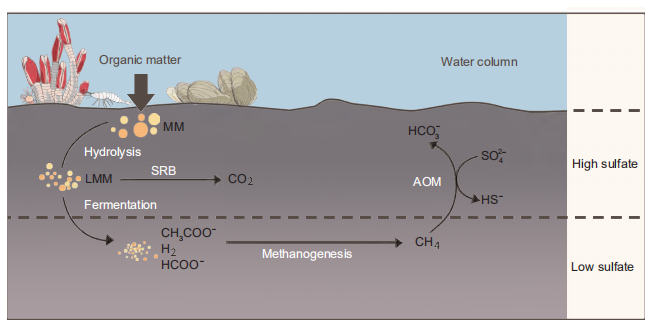
Figure 2 The organic carbon cycle derived by microorganisms in marine sediment. MM, macromolecules; LMM, low molecular weight molecules: SRB, sulfate reducing bacteria; AOM, anaerobic oxidation of methane.
Microbial organisms are the main producers and consumers at the bottom of the food chain. Methanogens, ANME and SRB are microorganisms that are directly involved in methane metabolism. A large amount of methane has been estimated to occur in sediments, and it is a strong greenhouse gas that has an important effect on global climate change. As the importance of methane in global climate change has been increasingly considered, microbial methane metabolism has become a hotspot in the study of the marine carbon cycle. With great progress in molecular biological approaches and the development of marine ecology, the study of methane metabolism in the ocean has made considerable progress. Summarizing the existing research results and research methods in this area can improve our understanding and is important for subsequent research.
In this review, we mainly focus on the introduction and summary of the studies and progress on the diversity and metabolic mechanisms of methane-metabolizing microbial organisms, and the effect of marine methane metabolism on climate change. The first part of this review introduces the diversity and distribution of methane metabolizing microbes, including their various metabolic features. Next, different pathways of methane production and oxidation are illustrated, as well as the impact of marine methane metabolic fluxes on climate change. Finally, we discuss new approaches and future directions in this field and our suggestions for development.
2. Methanogens and methane oxidizers
Two sources of methane are commonly recognized: micro-bial production “biogenic" and thermocatalytic methane “abiogenic” “Abiogenic” or "thermogenic" methane may be derived from heating of kerogen, pyrolysis or an inorganic reaction of water with hot ultramafic rocks and metals (Claypool and Kvenvolden, 1983). Biogenic methane is produced by microorganisms, which can utilize acetate, C1 compounds, CO2 and H2 to produce methane (Ferry and Lessner, 2008). Biogenic methane is the main source of marine methane.
2.1 Methanogenic microbial taxa
Methanogens are the microorganisms that produce methane as the end-product of their anaerobic respiration. All methanogens are strictly anaerobic archaea representing special lineages within the Euryarchaeota and are classified into four well-established classes: Methanomicrobia, Methanobacter- ia, Methanococci and Methanopyri. These classes are divided into seven orders: Methanococcales, Methanopyrales, Methanobacteriales, Methanosarcinales, Methanomicro- biales, Methanocellales and Methanomassiliicoccales (previously known as Methanoplasmatales).
The substrates for methanogens are limited to three major types: CO2, methyl-group containing compounds, and acetate. Although methanogens are very diverse, they can utilize only a restricted number of substrates. According to different types of substrates, methanogens can be assigned to three types: acetolactic, CO2 reducing and C1 compound-utilizing methanogens.
Most methanogens, including Methanococcales, Metha- nopyrales, Methanocellales, Methanobacteriales and Me- thanomicrobiales, can reduce CO2 to produce CH4 (Ferry and Lessner, 2008). For instance, Methanococcales can produce methane with CO2 as an electron acceptor and H2 or formates as electron donors. These microbes inhabit the marine environment and require sea salts for optimal growth (Liu and Whitman, 2008). Some members of Methanobacteriales can produce methane with formate, CO, or secondary alcohols as electron donors. Methanomicrobiales can use formate and secondary alcohols instead of hydrogen as electron donors to produce methane and are widely distributed in marine sediments, such as continental sediment, cold seep and hyperthermal vent ecosystems (Liu and Whitman, 2008). Methanocellales produce methane with formate and a low concentration of hydrogen. Methanocellales are distributed in rice fields, anaerobic freshwater and marine sediment (Lyu and Lu, 2015).
Methanogens that are able to use methylated compounds, or methylotrophic methanogens, are limited to the order Methanosarcinales. Methanobacteriales are hydro- genotrophic methanogens and are distributed in various anaerobic environments (marine or freshwater sediment, sewage, and animal gastrointestinal tracts) (Liu and Whitman, 2008).
Methanopyrales have only one species, Methanopyrus kandlerin, and can produce methane with CO2 and H2. This species is commonly found at high temperatures (84-110 C) and inhabits marine hydrothermal ecosystems (Huang et al., 2003; Itavaara et al., 2016; Lang, 2014; Nakagawa and Ta- kai, 2006; Paul et al., 2012; Poulsen et al., 2013; Takai and Horikoshi, 1999).
Methanomassiliicoccales (Methanoplasmatales) are hy- drogenotrophic methanogens that produce methane with methanol or methylamine and are distributed in various environments, including rice fields, marine thermal vents, and animal and human enterons (Egert et al., 2003; GroBkopf et al., 1998; Huang et al., 2003; Lang, 2014; Paul et al., 2012, Poulsen et al., 2013; Takai and Horikoshi, 1999).
Methanosarcinales contain various types of metabolic methane-producing pathways, including CO2 reducing, acetolactic methane-producing, and methylotrophic methanogens. Most members can produce methane by dis-proportionating methyl-group-containing compounds or by splitting acetate. Methanosarcinales contain two families, Methanosarcinaceae and Methanosaetaceae. All members of Methanosarcinaceae can produce methane by dis- proportionating methyl-group-containing compounds, while members of Methanosaetaceae can produce methane only by splitting acetate (Lee et al., 2011). These organisms are distributed in marine and anaerobic freshwater sediment and anaerobic sewage (Liu and Whitman, 2008).
2.2 Methane oxidizers
Methane can be aerobically and anaerobically oxidized to CO? by microbial organisms in marine sediment. Methano- trophic bacteria can convert methane to CO? only in the presence of oxygen (Hanson and Hanson, 1996). In this step, methane is the only carbon source and electron acceptor; oxygen is the electron donor. Methanotrophic bacteria are obligate aerobic bacteria and are widely distributed in methane-rich water, soil and interfaces between aerobic and anaerobic regions (Cicerone and Oremland, 1988). In marine environments, methanotrophic bacteria are mainly distributed in methane-rich water columns, interfaces between water and sediment or syntrophs with other marine invertebrates (Childress et al., 1986; Fisher et al., 1993). In anaerobic and nonmethane environments, methanotrophic bacteria form spores that can consist of unsuitable conditions (Hanson and Hanson, 1996).
Based on the intracellular structure and phylogeny and pathways of carbon metabolism, methanotrophic bacteria can be classified into two types一type I and type II (Green, 1992). The cell member of type I methanotrophic bacteria have many bundles of vesicular disks in the cell membrane and utilize ribulose monophosphate (RuMP) as the primary pathway to assimilate carbon. Many members of type I methanotrophic bacteria affiliate with Methanococcaceae (Gamma-Proteobacteria), including Methylobacter, Methy- lomonas, Methylomicrobium and Methylosphaera (Bratina et al., 1992; Hanson and Hanson, 1996). In contrast to type I, member of type II has paired membranes aligned to the periphery of cells and utilize the serine pathway to assimilate carbon (Hanson and Hanson, 1996). All type II methano- trophic bacteria affiliate with Alpha-proteobacteria and are represented by the family Methylocystaceae, including Me- thylocystis, Methylopila, and Methylosinus (Bratina et al., 1992; Hanson and Hanson, 1996). Members of the newly classified group, type X methanotrophic bacteria, are also affiliated with Gamma-proteobacteria and are similar to members of type I. Type X methanotrophic bacteria mainly include Methylococcus and Methylocaldum, and members of this group utilize RuMP and serine pathways and are widely distributed in high-temperature environments (Bratina et al., 1992). Compared to type I, type X methanotrophic bacteria contain high G+C contents in their DNA (Hanson and Hanson, 1996).
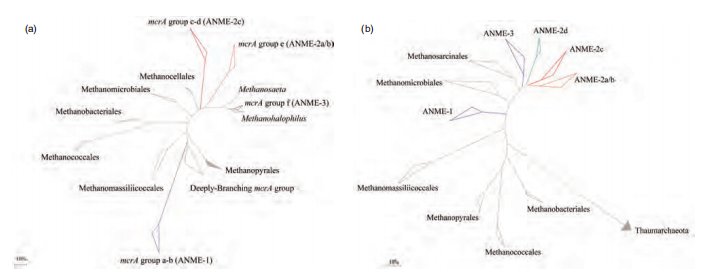
Figure 3 Phylogenetic trees showing the affiliations of ANME and methanogens based on mcrA (a) and 16S rRNA (b) gene sequences.
Anaerobic methanotrophs (ANaerobic MEthanotroph, ANME) are a type of archaea that can anaerobically oxidize methane to CO2, and this group belongs to Euryarchaeota. Based on the 16S rRNA gene phylogenetic analysis, ANME can be assigned to three different subgroups, ANME-1, ANME-2, and ANME-3. The similarity of the 16S rRNA gene among the three groups is 75-92% (Knittel and Boe- tius, 2009). ANME-2 and ANME-3 belong to Methano- sarcinales (Figure 3). ANME-1 is further phylogenetically related to Methanomicrobiales and Methanosarcinales (Knittel and Boetius, 2009). ANME-1 also has two subgroups: ANME-1a and ANME-1b. Furthermore, ANME-1b contains three different subgroups (ANME-1bI, II, III) (Niu et al., 2017). ANME-2 can be further divided into three subgroups: ANME-2a/b, ANME-2c, and ANME-2d (Me- thanoperedenaceae). The homology of the 16S rRNA gene among these subgroups is low (Haroon et al., 2013).
ANME-1 and ANME-2 archaea are usually associated with SRB of the Desufosarcina/Desufococcus clade (DSS; SEEP-SRB I) and affiliated with Deltaproteobacteria. ANME-1 cells most often occur as single cells or in chains of two to four cells to oxidize methane (Knittel et al., 2005; Reitner et al., 2005). ANME-2a and ANME-2c can be distinguished from each other by their aggregate morphology. ANME-2ab/Desufdsarcina (DSS) aggregates represent mixed-type aggregates. In Eel River Basin sediments, nearly equal numbers of ANME-2c/Desufdbulbus consortia and ANME-2c/DSS have been identified, indicating a versatility in bacterial partnership and AOM syntrophy. Typical ANME-2c/DSS aggregates represent the well-known shelltype with an inner core of ANME-2, which is partially or fully surrounded by an outer shell of SRB (Knittel and Boetius, 2009). The consortia formed by ANME-2/DSS are covered with thick silicate (Chen Y et al., 2015). ANME-3 archaea form shell-type aggregates with Desufobulbus-re- lated bacteria as a sulfate-reducing partner. At some sites, ANME-3 even occurs solely as single cells (Losekann et al., 2007; Niemann et al., 2006).
There is increasing evidence that the diversity of bacteria associated with ANME is not restricted to SRB. An analysis of ANME-2c consortia captured by whole-cell magnetofluorescence in situ hybridization (FISH) showed various bacterial partners coupled with ANME-2. Some studies have reported that ANME-2c or ANME-2a can aggregate with other bacteria, such as Sphingomonas spp. belonging to Al- pha-proteobacteria and Burkholderia spp. or Limnobacter spp. belonging to Beta-proteobacteria (Chen et al., 2016; Pernthaler et al., 2008). This evidence indicates that ANME may be coupled to the versatile metabolism or function of the bacterial partnership.
Since their discovery in the late 1990s, ANMEs have been found in marine sediment, and researchers have studied the distribution patterns of ANME in various environments (Hinrichs et al., 1999). ANMEs were found in different locations, such as terrestrial habitat, fresh water, and marine water and sediments, especially in methane-rich environments (e.g., mud volcanoes, cold seeps, sulfate-methane transition zone (SMTZs), and pockmarks) (Giovannelli et al., 2016; Nickel etal., 2012; Niemann et al., 2006; Shubenkova et al., 2010). ANMEs have also been found in the anaerobic chimneys and rocks of hydrothermal vents, and the water was found to contain soluble methane, even several kilometers below the seafloor (Ettwig et al., 2008; Inagaki et al., 2006; Raghoebarsing et al., 2006; Roussel et al., 2008; Schubert et al., 2006). The subgroups of ANME have different ecological niches and functions. ANMEs are often found in methane-rich environments and exhibit separation in different layers of sediments (Boetius et al., 2000; Hinrichs et al., 1999; Ruff et al., 2015). A study of archaeal diversity and distribution in the Haima cold seep found that ANME-2a/b was predominant in the upper and middle layers of the SMTZ, whereas ANME-1b outcompeted ANME-2 in the sulfate-depleted bottom layers of the SMTZ and the methanogenic zone (Niu et al., 2017).
The development of sequencing technology has allowed for metagenomic methods to be widely used in the research on culture-independent microbial ecology, providing insight into the diversity and evolution of methane-metabolizing microorganisms. Recently, researchers found many new ar- chaeal groups with potential methane-metabolized functions from different environmental samples. Evans et al. obtained two near-complete genomes (BA1 and BA2; the completeness of the genomes was 92% and 94%, respectively) belonging to the archaeal phylum Bathyarchaeota from formation water samples in the Surat Basin. Bathyarchaeota are a newly named archaeal phylum that might degrade organic matter in marine sediment (He et al., 2016; Lloyd et al., 2013; Meng et al., 2014). Surprisingly, the two genomes of BA1 (Bathy-3) and BA2 (Bathy-8) contain divergent homologs of the genes (mcrABGCD) necessary for methane metabolism, including those that encode the methyl-coen- zyme M reductase (MCR) complex and methyltransferases that are used in methyl compound utilization (mtsA, mtbA, mtaA, mttBC, and mtbBC). Furthermore, these genes have high similarity with the counterpart in obligate H2-utilizing methylotrophic methanogens belonging to the order Metha- nomassiliicoccales (Evans et al., 2015). Additional non- euryarchaeotal MCR-encoding genes identified in a range of environments suggested that unrecognized archaeal lineages may also contribute to global methane cycling. Using a similar approach, Vanwonterghem et al. recovered four nearcomplete archaeal genomes containing the mcrA gene from cellulose-degrading anaerobic digesters (Vanwonterghem et al., 2016). Three of these mcrA genes were found to have high similarity with already known methanogens. The other mcrA genes were found to be similar to those in the other three unknown methane-metabolizing groups. Based on mcrA gene and 16S rRNA gene analysis, these four genomes belong to a new archaeal phylum called Verstraetearchaeota, which are obligate H2-dependent methanogens that can utilize methyl compounds with hydrogen (Vanwonterghem et al., 2016).
3.The pathway of methane production and oxidization
3.1Pathway of methane production
Methanogens can use only limited substrates to produce methane, including CO2, H2, acetate, and C1-compounds. Methane is produced by three pathways: (1) reduction of carbon dioxide, (2) fermentation of acetate and (3) dis- mutation of methanol or methylamines. Many methanogenscan be isolated with various methods in the laboratory, and their metabolic mechanisms for methane production are well known. The three pathways of methane production are introduced as follows:
3.1.1Reduction of carbon dioxide
Most methanogens are hydrogenotrophic methanogens, which can use hydrogen as an electron donor to reduce carbon dioxide or formate as an alternative electron donor to produce methane (Table 1, reactions (1) and (2)).
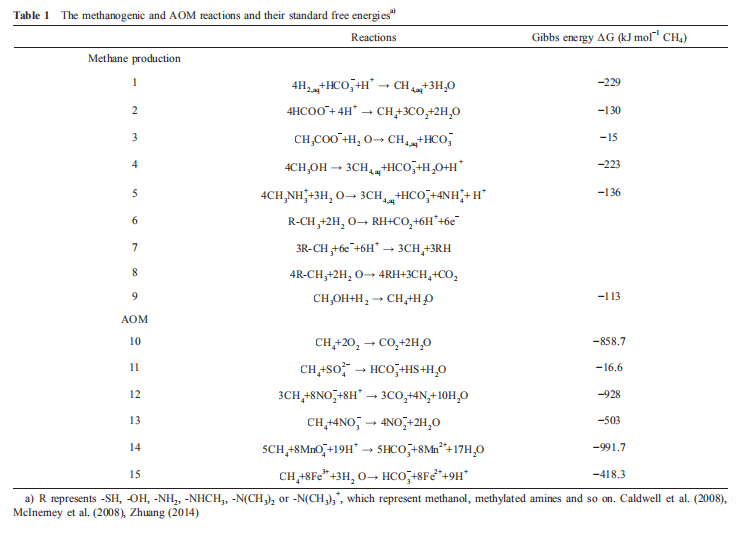
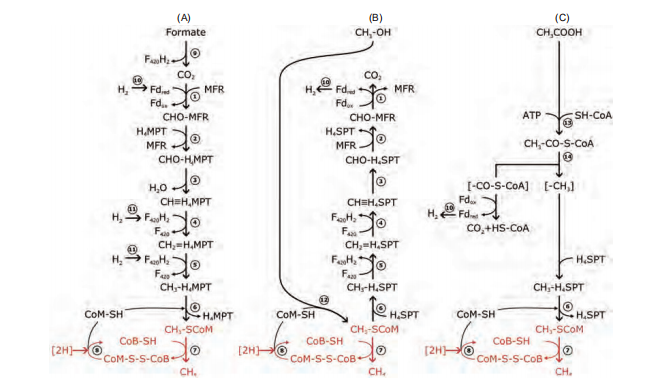
Figure 4 Pathways of methanogenesis. (A) Methanogenesis from H2/CO2 or formate. (B) Methanogenesis from methanol. (C) Methanogenesis from acetate. Common reactions in methanogenesis are labeled in red. ABBREVIATIONS: Fd?d reduced form of ferredoxin; Fdox oxidized form of ferredoxin; F420H2 reduced form coenzyme F420; MFR-methanofuran; H4MPT tetrahydromethanopterin; CoM-SH coenzyme M; CoB-SH coenzyme B; CoM-S-S-CoB heterodisulfide of CoM and CoB; CoASH coenzyme A. Enzymes: 1. formyl-MFR dehydrogenase (Fmd); 2. formyl-MFRiHqMPT formyltransferase (Ftr); 3. methenyl-HqMPT cyclohydrolase (Mch); 4. methylene-HqMPT dehydrogenase (Hmd)); 5. methylene-HqMPT reductase (Mer); 6. methyl-HqMPPHS-CoM methyltransferase (Mtr); 7. methyl-CoM reductase (Mcr); 8. heterodisulfide reductase (Hdr); 9. formate dehydrogenase (Fdh); 10. energyconserving hydrogenase (Ech); 11. F42 -reducing hydrogenases; 12. methyltransferase; 13. acetate kinase (AK)-phosphotransacetylase (PTA) system in Methanosarcina; AMP-forming acetyl-CoA synthetase in Methanosaeta; 14. CO dehydrogenase/acetyl-CoA synthase (CODH/ACS).
CO2 reduction in the methane-producing pathway mainly consists of seven steps (Figure 4) and an additional branch: CoM-S-S-CoB reduction catalyzed by heterodisulfide reductase (Hdr). CO2 is successively reduced to methane through the formyl, methylene, and methyl levels. Metha- nofuran (MFR) and tetrahydromethanopterin (H4MPT) are the main methyl vectors. Initially, CO2 binds to MFR and is reduced to the formyl group. The formyl group is then transferred to H4MPT to form formyl-H4MPT. The formyl group is then dehydrated to methylene-H4MPT and then to methyl-HqMPT. Reductive coenzyme F420 (F420H2) is an electron donor in steps 4 and 5. Reduced F420 (F420H2) is the direct electron donor in these two reduction steps. The me-thyl group is then transferred to CoM, forming methyl-CoM. The last reduction step reduces methyl-CoM to MCR, which is the key enzyme in methanogenesis. Coenzyme B (CoB) is the direct electron donor in this reduction, and the oxidized CoB forms a heterodisulfide with CoM (CoM-S-S-CoB), followed by the reduction of heterodisulfide to regenerate the thiols (Ferry, 2010). The methyl transfer reaction is catalyzed by methyl-H4MPT:HS-CoM methyltransferase (Mtr), which is a membrane-bound complex (Lessner, 2001). Both reactions一the methyl transfer from H4MPT to CoM and the reduction of heterodisulfide一are exergonic and involved in energy conservation.
3.1.2Fermentation of acetate
Acetate is a very active intermediate compound in the process of anaerobic organic oxidation that eventually leads to the mineralization of organic matter in sediment and is also an important substrate for methane production (McInerney et al., 2008). Methane production with acetate occurs via aceticlastic pathways (Liu and Whitman, 2008). The carboncarbon bond of acetate is cleaved, the methyl group reduces to methane, and the carboxyl-group oxidizes to CO2. First, acetyl-CoA aligning to the carbonyl part of acetate is cata-lyzed by acetate kinase and phosphotransacetylase. The C-C bond of carbon monoxide, the C-S bond of coenzyme A and the acetyl group are cleaved by the dehydrogenase/acetyl- CoA synthase (CODH/ACS) complex (Gong et al., 2008). The carbonyl group is converted to CO2 with ferredoxin to release extracellularly and change to carbonate with Cam (carbonic anhydrase) (Fischer and Thauer, 1990). The methyl group is then transferred to H4SPT and then HS-CoM with MTR, which is a member-bound protein complex. Finally, the methyl group of acetate is converted to CH4 and transfers electrons to CoM-S-S-CoB, which is the terminal electron acceptor of a membrane-bound electron transport chain coupled to the formation of an electrochemical proton gradient that drives adenosine triphosphate (ATP) synthesis. CoM-S-S-CoB is catalyzed to HS-CoB and HS-CoM by heterodisulfide reductase (Hdr). The reduction of CoM-S-S- CoB is shown in Figure 4 (Maupin-Furlow and Ferry, 1996).
3.1.3Dismutation of Cl compounds
The C1 compounds contain methanol, methylated amines (monomethylamine, dimethylamine, trimethylamine, and tetramethylammonium), and methylated sulfides (methanethiol and dimethylsulfide). Methylotrophic methanogens can use these substrates to produce methane, and all are affiliated with the order Methanosarcinales, except for Me- thanosphaera species, which belong to the order Methano- bacteriales. In methylotrophic methanogenesis, the methyl groups from methylated compounds are activated and divided into two parts: methyl groups and other residues. The activation and transfer of the methyl group require substratespecific methyltransferases. Methyl groups are transferred to a cognate corrinoid protein and then to CoM. With the electrons from the oxidation of other residues as the reverse of hydrogenotrophic methanogenesis to CO2, methyl-CoM is reduced to methane catalyzed by MCR.
The conversion of methanol and methylamines to methane and carbon dioxide is a dismutation reaction in which the methyl group of one substrate molecule is oxidized to carbon dioxide, providing six electrons for reduction of the methyl groups of the other three substrate molecules to methane (Table 1, reaction (6)). Methanomicrococcus blatticola and Methanosphaera methanogens are H2-dependent methylo- trophic methanogens that utilize only methyl and H2 to produce methane (Table 1, reaction (9); Sorokin et al., 2017). Recently, Sorokin et al. (2017) isolated two pure cultured methanogens from hypersaline environments. These methanogens are heterotrophic methyl-reducers that use C1-me- thylated compounds as electron acceptors and formate or hydrogen as electron donors. The genomes contain an incomplete and apparently inactivated set of genes encoding the upper branch of methyl group oxidation to CO2 as well as membrane-bound heterodisulfide reductase and cytochromes. These features differentiate “Methanona- tronarchaeia" from all known methyl-reducing methanogens. The discovery of extremely halophilic, methyl-reducing methanogens related to Haloarchaea provides insights into the origin of methanogenesis and shows that the strategies employed by methanogens to thrive in salt-saturating conditions are not limited to the classical methylotrophic pathway (Sorokin et al., 2017).
Generally, marine methanogens mainly use CO2,H2 and acetate to produce methane. However, methane production with Cl-methylated compounds is underestimated. Recently, several studies have reported that some C1-compounds, including methanol, methylated amines and dimethylsulfide, are widely distributed in marine sediment. Methane production with C1-methylated compounds may be an important methane-producing process in marine sediment (Zhuang et al., 2018, 2017, 2014, 2016). For instance, Cl compounds are rich in hyper-saline environments where methylotrophic methane production is predominant (Zhuang et al., 2016). Alternatively, in a sulfate environment, me- thylotrophic methanogens can utilize C1-compounds to produce methane, as SRB rarely use these compounds (Mitterer, 2010; Xiao et al., 2017; Zhuang et al., 2017).
3.2 Methane oxidization
3.2.1 The pathway of aerobic methane oxidization
Methane can be oxidized to CO2 aerobically by microbial organisms in sediment, where oxygen is present, such as the surficial layer of sediment, interface of water and sediment, the water column or inside of some syntrophic animals (Conrad, 2009; Knief, 2015; Reeburgh, 2007). Many kinds of bacterial groups are involved in aerobic methane oxidation, which are derived from 18 genera of Deltaproteo- bacteria and 5 genera of Alpha-proteobacteria (Knief, 2015). At the surface of the seabed, methane that erupts from the deep sediments is oxidized by methanotrophic bacteria in aerobic environments. Because oxygen is exhausted quickly by fauna in the surficial sediment layer, most marine sediments are anaerobic. In marine environments, compared to anaerobic methane oxidation, aerobic methane oxidation accounts for small parts of methane consumption (Boetius and Wenzhofer, 2013).
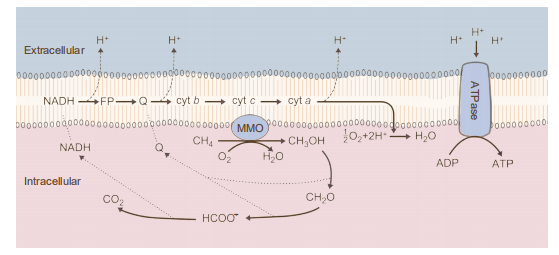
Figure 5 Pathways for the oxidation of methane and assimilation of formaldehyde. Abbreviations: Cyt c, cytochrome c; FADH, formaldehyde dehydrogenase; FDH, formate dehydrogenase
In 1906, Sohngen et al. isolated the first methane-oxidizing bacterium, and since that time, scientists have obtained many pure cultured aerobic methanotrophic bacteria (Sohngen, 1906). The metabolic mechanism of aerobic methane oxidation has been studied directly. The oxidation of methane by aerobic methanotrophs is initiated by methane monooxygenases (MMOs), which are classical monooxygenases that utilize two reducing equivalents to split the O-O bonds of dioxygen. One of the oxygen atoms is reduced to form H2O, the other is incorporated into methane to form CH3OH, and then, these compounds are oxidized to formaldehyde by a periplasmic methanol dehydrogenase (Lipscomb, 1994). Formaldehyde can be oxidized to formate by multiple enzyme systems. In the last step, formate is oxidized to CO2 by NAD-dependent formate dehydrogenase in most methano- trophs (Table 1, reaction (10)). Typical redox cofactors, such as quinones and NADH, are oxidized and enter the electron transfer chain of the cell membrane to drive active protons through membrane-bound ATP synthase to produce ATP (Figure 5; Hanson and Hanson, 1996).
Two forms of MMOs have been found in methanotrophic bacteria. One form is a soluble MMO (sMMO), and the other is a particulate or membrane-bound MMO (pMMO) (Smith and Dalton, 1989). All known methanotrophs are capable of forming pMMO when grown in the presence of copper. The gene encoding pMMO can be used as a probe for metha- notrophic bacteria (Dalton, 1992). In the absence of copper, some methanotrophic bacteria can form sMMO, which utilizes NADH+H as an electron donor (Lipscomb, 1994). sMMO is mainly synthesized in type II and type X metha- notrophic bacteria, while it is rarely found in type I. Most type I methanotrophs require higher levels of copper for growth than required by type II methanotrophs and Methy- lococcus capsulatus (type X) presumably because they require increased levels of copper for pMMO activity and lack the genetic information for sMMO synthesis (Graham et al., 1993; Murrell, 1994). The synthesis of sMMO by some methanotrophs may be a survival mechanism in environments where the copper concentration limits the growth of methanotrophs that synthesize only pMMO (Hanson and Hanson, 1996).
Under aerobic conditions, methane is oxidized to methanol, which is subsequently oxidized to formaldehyde by dehydrogenase. Most of the reducing power required for the metabolism of methane is produced by the oxidation of formaldehyde via formate to carbon dioxide. Formaldehyde is oxidized to formate through two pathways, the RuMP and serine pathways (Hanson and Hanson, 1996). The RuMP pathway is very efficient and utilizes RuMP for formaldehyde assimilation. However, the serine pathway requires more ATP and extra reducing power (Hanson and Hanson, 1996).
3.2.2 The pathway of anaerobic methane oxidization Methane oxidation is generally considered to be reverse methanogenesis.
In contrast to aerobic methane oxidation, a large amount of methane is consumed by anaerobic methane oxidation (Knittel and Boetius, 2009). Although anaerobic methane oxidation occurs in various environments, such as in anaerobic freshwater and marine sediments, wetlands, and anaerobic sewage, pure cultured ANMEs have not yet been obtained (Knittel and Boetius, 2009; Knittel et al., 2005). The mechanism of anaerobic oxidation of methane remains enigmatic. However, studies combining omic, FISH-nano- SIMS, SIP and enzymology have helped better define the mechanism of AOM and interactions between ANME and SRB.
Nickel-containing MCR is the key enzyme in biological methane formation by methanogenic archaea (Scheller et al.,2010). Indirect evidence indicates that the anaerobic oxidation of methane might proceed as the reverse of archaeal methanogenesis from carbon dioxide with the nickel containing MCR as the methane activating enzyme (Shima et al., 2012). Homologs of MCR have been discovered in microorganisms carrying out the opposite process-AOM catalyzed the first step. The structure of MCR homologs of ANME-1 is similar to MCR of methanogens and contains coenzyme M and coenzyme B. The difference is that MCR of ANME-1 has a F430 variant, a cysteine-rich patch and an altered post- translational amino acid modification pattern, which may make the enzymes adapt to different biological contexts (Shima et al., 2012). Although the structure and function of the enzyme are still not clear, F430 modification and cysteine- rich patches may be considered a redox-dependent electron/ electron transformation system, and this striking cysteine- rich patch proposed as a redox-relay system might be used for the reduction of ANME-1 MCR from the inactive Ni2+ to the active Ni+ oxidation state.
In addition, a metagenomics analysis of marine sediment indicated that all key functional enzymes in the methanogenesis pathway are anaerobic methanotroph—ANME-1, except MER (Hallam et al., 2004). Single-cell sequencing and metatranscriptomic analysis of enriched mud volcano sediments (ANME-2a are the main archaeal groups in AOM) indicated that all enzymes involved in CO2 reducing methanogenesis are found in the ANME-2a genome and are actively expressed (Wang et al., 2014; Zhang et al., 2010,
2011). This evidence indicates that ANME archaea anaerobically oxidize methane to CO2 by very similar enzymes and essentially the same catalytic steps as methanogens that reduce CO2 to methane but in the reverse direction.
Although studies on the mechanisms of AOM have progressed, there are some questions that remain unknown, such as whether different groups of ANME utilize the same or similar mechanisms of AOM and how electron transfer is involved in AOM and energy metabolism in ANME cells. One of the research hotspots is the identification of the key intermediates in AOM. Some studies proposed that the intermediates might be hydrogen, acetate, methyl sulfur and zero-valent sulfur (Hoehler et al., 1994; Meulepas et al., 2010; Milucka et al., 2012; Moran etal., 2008; Valentine and Reeburgh, 2000). The most recent studies have shown that direct electron transfer might be an important mechanism between ANME and SRB. McGlynn et al. examined the influence of interspecies spatial positioning as it relates to biosynthetic activity within structurally diverse uncultured methane-oxidizing consortia, ANME and SRB (ANME-2c: SEEP-SRB1a and ANME-2c or ANME-2b: Deltaproteo- bacteria). This influence was determined by measuring stable isotope incorporation for individual archaeal and bacterial cells to constrain their potential metabolic interactions with FISH and NanoSIMS. The results demonstrated that the activity of cells was not related to the distance between cells, and cytochrome was found in consortia of ANME and SRB with histochemical staining (3,3'-diaminobenzidine, DAB). Combined with the detection of large multi-haem cytochromes in the genome of methanotrophic archaea and the demonstration of redox-dependent staining of the matrix between cells in consortia, these results provide evidence of syntrophic coupling through direct electron transfer (McGlynn et al., 2015). Other scientists also found the genesencoded haem cytochrome in the ANME-2 genome (Haroon et al., 2013; Meyerdierks et al., 2010; Wang et al., 2014). These findings were deduced and simulated using a model of direct electron transfer in the consortia.
Multi-species aggregates participating in direct interspecies electron transfer are possible with conductive nanowires. Wegener et al. incubated the thermophilic AOM consortia, which were mainly composed of ANME-1 and HotSeep-1, with hydrogen as the only electron donor (Wegener et al., 2015). Although the sulfate reducer effectively uses hydrogen as an electron donor, slow rates of hydrogen production by the consortium and a lack of hydrogenase gene sequences in the ANME-1 genome suggest that hydrogen is not the interspecies electron carrier (Wegener et al., 2015). Based on metagenome and metatranscriptome analysis, the gene encoding extracellular multi-haem cytochrome c protein was found in the ANME-1 genome, while genes encoding the synthesis and assembly of cilia and extracellular haem cytochrome c protein were found in HotSeep-1. Both partners greatly increase the expression of genes for outer surface c-type cytochromes when cooperating to oxidize methane. Furthermore, the sulfate reducing bacteria also highly expresses genes for type IV pili when grown syn- trophically, but not when grown on hydrogen (Wegener et al., 2015). Thus, when hydrogen is an electron donor, the expression of genes encoding cytochrome is down-regulated in ANME cells and HotSeep-1 cells. Abundant pili are apparent with transmission electron microscopy in aggregates oxidizing methane (Wegener et al., 2015). The conductivity of the pili or the aggregates was not measured, but it was speculated that the pili might be electrically conductive in a manner similar to Geobacter e-pili (Wegener et al., 2015). Alternatively, Scheller et al. used AQDS (9,10-anthraqui- none-2,6-disulfonat, a soluble artificial oxidant) anabolically decoupled from their syntrophic SRB partners. ANMEs still sustains high rates of methane oxidation when grown with AQDS instead of sulfate. Furthermore, other electron acceptors, including AQDS isomers, humic acids, and iron (III) complexes, have the ability to accept single electrons. Me- thane-metabolizing microbes that have been found in wetlands can utilize natural organic matter as electron acceptors during AOM (Valenzuela et al., 2017). These results suggest that direct electron transfer is a principal mechanism in AOM, which may also explain the enigmatic functioning and specificity of other methanotrophic ANME-SRB consortia.
AOM coupled to sulfate reduction has been most extensively studied because of the abundance of sulfate in marine systems, but electron acceptors other than sulfate are more energetically favorable than sulfate. Methane can be oxidized with other electron acceptors, such as iron, nitrate and nitrite. Anaerobic oxidation of methane coupled to denitrification of nitrate (nitrate/nitrite-dependent AOM, N- AOM) was first found in fresh-water sediment (Raghoebarsing et al., 2006). The N-AOM reaction is mediated by microbial consortium-coupled anaerobic methane oxidation to denitrification with nitrate or nitrite as electron acceptors (Table 1, reactions (12) and (13); Raghoebarsing et al., 2006). Further studies found that some specific bacteria could oxidize methane coupled to the complete denitrification of nitrite/nitrate to N? and called “Candidatus Methy- lomirabilis oxyferd'. Genomic analysis showed that Methylomirabilis oxyfera also had all genes involved in aerobic methane oxidation, including the gene (pmoA) encoding pMMO and denitrification, lacking the nosZDFY gene encoding the enzyme that catalyzes NO? to N?, and genes encoding the homolog of MCR (Ettwig et al., 2010, 2008, 2009). Isotope experimental results illustrated that nitrite could be reduced to NO; and then, two molecular NO could be converted to N2 and O2, which could activate pMMO to oxidize methane (Table 1, reaction (12)). Eight molecular nitrites produce four molecular O2, three of which can oxidize methane, and the remaining O2 is utilized by membrane-bound bo-type terminal oxidase (Wu et al., 2011).
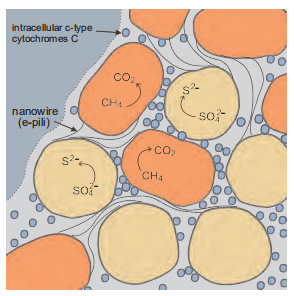
Figure 6 Model for direct interspecies electron transfer in consortium of ANME-2 (orange) and syntrophic sulfate-reducing partner (yellow) anaerobically oxidizing methane with the reduction of sulfate. Cytochrome (hexagons, diamonds and crosses)-based electron transfer proposed for adjacent cells (blue dots) and nanowires (black line) proposed for electron transfer to non-adjacent cells.
In the N-AOM enrichment, in addition to M. oxyfera, the abundance of a new subgroup of ANME, ANME-2d (Ca. Methanoperedens nitroreducens) increases significantly. Based on the metabolic reconstruction of the obtained Methanoperedens nitroreducens genome, a complete reverse methanogenesis pathway including all mcr subunit genes (mcrABCDG) and F42 -dependent 5, 10-methenyltetrahy- dromethanopterin reductase (mer) gene were identified, and reverse methane production was first found in M. nitror- educens. The nar genes related to nitrate reduction were identified in the genome, which was acquired laterally from a bacterial donor. All necessary genes for AOM and nitrate reduction were highly expressed, and no other genes or transcripts for anaerobic or aerobic methane oxidation were detected in the metagenome or metatranscriptome. Based on isotopic experiments and metatranscriptome analysis, the Kuenenia anammox population was identified as the consumer of nitrite produced by "M. nitroreducens”. In the presence of ammonium, ANME-2d couples AOM to reduce nitrate to nitrite, which could be converted to N2 by anaerobic ammonium oxidizers. In the absence of ammonium, ANME-2d couples AOM to reduce nitrate to nitrite, which is converted to N2 by NC10 (Figure 6; Haroon et al., 2013).
In the freshwater environment, especially in the oxygen and nitrate exhausting zone and deep anaerobic sediments, methane is mainly exhausted with N-AOM (Chen J et al., 2015; Deutzmann et al., 2014; Ettwig et al., 2010, 2016; Raghoebarsing et al., 2006). Recent research indicated that many types of NC10 groups were found in marine oxygen minimum zones (OMZ) and sediments, which indicates that N-AOM might occur in marine environments (Padilla et al., 2016). N-AOM is important for estimating methane oxidization and methane metabolic cycling, although the me-chanisms of N-AOM have not yet been clarified.
In addition to inorganic salts, large amounts of metal irons have been moved to continental margins from rivers. Irons are alternative electron acceptors for organic matter oxidization. Large amounts ofMn4+ and Fe3+ in marine sediments could technically be the electron acceptors in AOM and produce CO2 and iron sulfide (Beal et al., 2009). Some studies found iron sulfide in sediments of cold seeps. This evidence indicated that iron could be an electron acceptor in AOM. Beal et al. utilized birnessite and ferrihydrite as electron acceptors to incubate sediment from cold seeps, and the results showed that microorganisms from marine me- thane-seep sediment are capable of using manganese (bir- nessite) and iron (ferrihydrite) to oxidize methane, revealing that marine AOM is coupled, either directly or indirectly, to a larger variety of oxidants than previously thought (Beal et al., 2009). These reactions are listed in Table 1, reactions (14) and (15). However, there was no evidence for AOM in the presence of ferric oxyhydroxide, indicating that microorganisms responsible for AOM might be specific to the different iron compounds. An interesting finding is that the microorganisms responsible for ferrihydrite-dependent AOM have the potential to receive energy at approximately twice the rate of sulfate-dependent AOM, although that they are oxidize methane at approximately one-tenth the rate (Beal et al., 2009).
Some aerobic methane-oxidizing bacteria might be involved in iron-dependent AOM in freshwater sediment where iron is present. Baror et al. (2017) incubated lake sediments with different iron oxides, such as magnetite and hematite, and 13C-labeled methane. The results showed that strong uptake of 13C into the fatty acids of methanotrophic bacteria, was associated with increasing copies of the functional methane monooxygenase pmoA gene. This result indicated that archaea are not directly involved in full methane oxidation, but their participation is crucial, possibly as mediators in electron transfer.
These findings suggest that the mechanism of iron-coupled AOM is accomplished by a complex microbe-mineral reaction network, which is likely representative of many similar but hidden interactions sustaining life under highly reducing low energy conditions. Large amounts of manganese and iron are provided to oceans from rivers, indicating that manganese- and iron-dependent AOM have large potential effects on the AOM globally (Baror et al., 2017; Beal et al., 2009).
4.Effects of global climate change on marine methane-metabolizing pathways in marine sediments
A large amount of methane has been preserved in marine continental sediments; most of which has formed gas hydrate. Estimating the global inventory of methane in marine sediments is very difficult due to limited drilling data from the gas hydrate regions and dependence on diverse physical parameters (Buffett and Archer, 2004; Milkov, 2004; Pinero et al., 2013; Wallmann et al., 2012). Based on new models, the inventory of methane hydrates in marine sediments was 500-2000 Gt of carbon (Wallmann et al., 2012). Because temperature and pressure increase with sediment depth, gas hydrate degrades to methane gas, and under high pressure, this methane erupts to the surface via pores or sediment fractures, forming methane seeps and vents (cold seep, mud volcano, pockmark). Some parts of methane gas can dissolve in seawater and be released into the atmosphere. Methane is a strong greenhouse gas, which has an effect that is 23 times higher than that of CO2 (Qjima et al., 1993). Much of the methane released into the atmosphere could warm the global climate and result in accelerated degradation of methane hydrates in marine sediment. Therefore, research on methane oxidization and transfer mechanisms and estimations of the gas hydrate inventory are critical for global climate change.
Methane seeps in continental slope sediments are formed by decomposing gas hydrate in deep sediment. Based on estimations, the total efflux of methane to the overlying ocean could range from 0.01 to 0.05 Gt of carbon annually, accounting for 1-5% of the methane in the atmosphere (Dickens, 2003; Reeburgh, 2007). Methane can escape from sediment to seawater, and more than 80% of methane is oxidized anaerobically by ANME and SRB in deep sediment. The remaining methane (approximately 0.02 Gt C yr-1) is oxidized anaerobically or aerobically byANME and methanotrophic bacteria at the interface between water and sediments. Finally, the remaining methane that is released into the atmosphere is very limited (Figure 7; Boetius and Wenzhofer, 2013; Lesniewski et al., 2012).
The microbial methane metabolism rate is an important factor in methane leakage, which is essentially controlled by the following key factors: the flow velocity in seep areas; the abundance and activity of methanotrophic microorganisms; and the pathway of methane transformation (Niemann et al., 2006; Sommer et al., 2006).
The mechanisms and magnitude of the biological sink for methane in subsurface sediments represent significant uncertainty in the marine methane budget. The consumption of methane by seafloor biota is termed the benthic filter for methane and influences the amount of methane emitted from the sea floor, mainly including aerobic and anaerobic methane oxidization (Boetius and Wenzhofer, 2013). In deep marine sediments, most methane is exhausted by AOM, and the unknown fraction that escapes methane flux and reaches the hydrosphere will be substantially consumed by aerobic methanotrophs. The velocity of the upward methane flux is a significant factor for methane oxidization and influences the amount of methane emitted from the sea floor. The efficiency of this filter can be quantified as the proportion of total methane oxidized (i.e., half the total oxygen uptake) to total methane flux (efflux plus oxidation) (Boetius and Wenzho- fer, 2013). The efficiency of the benthic methane filter at cold seeps is reduced from approximately 80% in systems with low fluid flow to approximately 20% in systems with intermediate flow (Boetius and Wenzhofer, 2013).
In most continental sediments, methane is predominantly consumed by ANME in the SMTZ. In cold seep areas, the methane concentration is high, and methane-metabolizing microorganisms are highly abundant and active. The rate of AOM in a cold seep is much higher than that in a diffusion area. The rate of AOM is 3-150 mmol L 1 day 1 m 2 (Boe- tius and Wenzhofer, 2013). In the sediment outside of seep areas, methane flux to the hydrosphere is almost fully controlled by benthic microbes in diffusion-dominated SMTZs. Based on estimations, the amount of methane oxidation is 0.05 Gt C yr-1 in the diffusion-dominated SMTZs of continental slope sediments (assuming the area of continental slope is 4.1x107 km2, the rate of AOM is 0.3 mmol C m-2 d-1) (Boetius and Wenzhofer, 2013).
The abundance of fauna and microorganisms influence the efficiency of the benthic methane filter. The efficiency of the benthic filter for methane is less than 10% in areas where fauna and bacterial mats are absent and where strong gas ebullition occurs, resulting in increased methane escape to the hydrosphere and atmosphere (Boetius and Wenzhofer, 2013; Niemann et al., 2006).
In addition to dissolved methane efflux, methane can be transferred in bubble form which is difficult to quantify at seeps. In the form of gas bubbles, methane gas is hardly consumed by benthic microbes (Romer et al., 2012). Most active seeps release large numbers of methane gas bubbles into the water column, which are also called “gas plumes". Regional surveys with multibeam and single-beam hydroacoustics combined with visual observations of the sea floor have been used to quantify the free methane gas erupting from cold seeps at the sea floor. Individual flares can release 10-100 t C yr-1 into the hydrosphere (Valentine et al., 2001). However, bubbles emitted from seeps over 1500 m deep are unlikely to reach the atmosphere. Based on observations, gaseous methane emissions from single submarine mud volcanoes have been estimated to be on the order of a few thousand t C yr-1. Some of these highly active seeps may contribute to atmospheric methane emissions (Romer et al.,
2012).
Shallow marine sediments in the Arctic and Antarctic Oceans are potential methane reservoirs that preserve large quantities of methane stored in hydrates and submerged permafrost (Blake et al., 2015; Enzmann et al., 2018; McCalley et al., 2014). Because the physical and biogeochemical processes in the polar regions are distinguished from those in other ocean areas, in addition to the factors mentioned above, there are characteristic factor that influence the regulation of methane oxidization, including increased freshwater discharge and ice cover (James et al., 2016). In winter, ice cover on the surface of the water column has the potential for major restrictions and restricted free airsea exchange, which will also trap methane bubbles reaching the surface and result in high methane concentrations under ice. For instance, on the East Siberian Arctic shelf, dissolved methane concentrations beneath the sea ice are higher than those in winter (Shakhova et al., 2010), and methane oversaturation has been found under multi-year sea-ice in the Canadian Arctic (Kitidis et al., 2010). Similar to global warming, continuous sea-ice melting will give rise to dramatic increases in methane transport into the atmosphere (He et al., 2013). Alternatively, in Arctic shelf seas, the freshwater derived from ice melting and river runoff may increase the stratification of water and inhibit the transfer of methane gas to surface waters (Rudels et al., 1991; Semiletov et al., 2000).
5.Outlook
5.1 Discovering new species of methanogens and me- thanotrophs
The deep sea is an extreme environment with low temperatures and high pressure, and it has physiochemical parameters that are different from those in other ecosystems. Some unknown methane-metabolizing microbes may be found in the deep sea, especially in a methane-rich en-vironment, where new methane consumers could be isolated and easily discovered. For instance, Ken Takai et al. isolated a thermophilic methanogen from a hydrothermal vent (Takai et al., 2008). With the development and application of single cell sequencing, metagenomics, metatranscriptome and metaproteomics, independent-cultured microbial methods might be used to find other function-unknown archaea that may be potential methane consumers.
5.2The mechanisms of anaerobic methane oxidation
Since 1994, anaerobic oxidation of methane has been found in marine sediment, and the mechanism of AOM has remained enigmatic. Several biochemical pathways for AOM have been proposed, including reverse methanogenesis, acetogenesis, and methylogenesis, and both culture-dependent and culture-independent techniques have provided some clues. Nevertheless, most questions remain unknown regarding the diversity, physiology, and metabolic restrictions of AOM-related organisms. To study the mechanisms of AOM, some scientists have tried to enrich these microbes with different instruments. Valentine et al. devised a microbial culture apparatus capable of maintaining subnanomolar H2 concentrations. This apparatus provides a method for studying interspecies hydrogen transfer by externally fulfilling the thermodynamic requirement for low H2 concentrations, thereby obviating the need for the use of cocultures to study some forms of metabolism (Valentine et al., 2000). Zhang et al. stimulated in vitro SR-AOM activity and designed a (continuous) high-pressure bioreactor system. Fed-batch mode incubations showed that the elevated methane pressure stimulated SR-AOM activity (Wang et al., 2014; Zhang et al., 2010). An apparatus that stimulates the in situ environment could contribute to enriching ANME in the laboratory. Further analysis integrating FISH, single cell sequencing, metagenomics, metatranscriptomics, stable isotope probes, and biomarkers may promote studies about the mechanism of AOM.
5.3AOM in biogeochemistry
The anaerobic oxidation of methane is a very important biogeochemical process in the ocean. The anaerobic oxidation of methane forms large amounts of carbonates and sulfides. The formation of authigenic carbonates and isotopes of different elements in sulfides can reveal the formation of oceans and geological changes at geological time scales (Feng et al., 2009; Tong et al., 2013). The composition of stable carbon isotopes in carbonates at cold seeps can reveal the sources of methane, and oxygen isotope changes can reflect the composition and temperature of methane fluids during carbonate formation (Feng et al., 2009; Tong et al., 2013). Fully researching information on the isotopic composition changes in these autogenous carbonates and sulfides and combining the changes in isotope composition of different chemical elements in sediments and pore waters will help us deduce the geological events that occurred during geological formation and the formation of oceans. It is important for us to understand the process of Earth formation.
5.4Estimating the methane inventory
Gas hydrate is a dynamitic methane reservoir in marine sediment. Surveying and estimating the amount of methane that is released into the atmosphere could substantially contribute to global climate change. It is very challenging to estimate the methane inventory. Some studies have used different mathematical models to estimate the reserves of methane in global marine sediments. However, due to the limitations of model parameters and the lack of long-term observations and quantitative studies of the global methane seep system, the values of methane reserves estimated by different models are very divergent (Pinero et al., 2013; Wallmann et al., 2012). Therefore, we need to develop models based on different geological environments and obtain data on long-term observations and quantitative monitoring of multiple cold seeps. In particular, the aerobic and anaerobic oxidation rates of methane, the bubble transfer process, and geological characteristics have significant effects on methane consumption and are fundamental parameters in the construction of models. Alternatively, because many studies have shown that other electron acceptors, such as nitrate, nitrite, iron and magnesium, could couple to AOM, these mechanisms should be considered in new models. The combination of optimized mathematical models and metadata from geological surveys may eventually produce more accurate estimations of the methane reserves in seabed sediments and their contribution to global climate change.
Acknowledgements The authors would like to thank Dr Guangchao Zhuang from Georgia University, Dr. Yinzhao Wang, Lei Xu and Lewen Liang from Shanghai Jiao Tong University for providing several helpful comments and suggestions. This work was supported by the State KeyR & D Project of China (Grant No. 2016YFA0601102) and the National Natural Science Foundation of China (Grant Nos. 41525011, 91228201 & 91428308) and the National Special Project on Gas Hydrate of China (Grant Nos. GZH201100311 & DD20160217). This study is also a contribution to the international IMBER project.
References
Baror I, Elvert M, Eckert W, Kushmaro A, Vigderovich H, Zhu Q, Ben- Dov E, Sivan O. 2017. Iron-coupled anaerobic oxidation of methane performed by a mixed bacterial-archaeal community based on poorly reactive minerals. Environ Sci Technol, 51: 12293-12301
Beal E J, House C H, Orphan V J. 2009. Manganese- and iron-dependent marine methane oxidation. Science, 325: 184-187
Blake L I, Tveit A, 0vreas L, Head I M, Gray N D. 2015. Response of methanogens in arctic sediments to temperature and methanogenic substrate availability. PLoS One, 10: e0129733
Boetius A, Ravenschlag K, Schubert C J, Rickert D, Widdel F, Gieseke A, Amann R, Jorgensen B B, Witte U, Pfannkuche O. 2000. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature, 407: 623-626
Boetius A, Wenzhofer F. 2013. Seafloor oxygen consumption fuelled by methane from cold seeps. Nat Geosci, 6: 725-734
Bratina B J, Brusseau G A, Hanson R S. 1992. Use of 16S rRNA analysis to investigate phylogeny of methylotrophic bacteria. Int J Syst Bac- teriol, 42: 645-648
Buffett B, Archer D. 2004. Global inventory of methane clathrate: Sensitivity to changes in the deep ocean. Earth Planet Sci Lett, 227: 185-199
Burdige D J. 2007. Preservation of organic matter in marine sediments: Controls, mechanisms, and an imbalance in sediment organic carbon budgets? Chem Rev, 107: 467-485
Caldwell S L, Laidler J R, Brewer E A, Eberly J O, Sandborgh S C, Colwell F S. 2008. Anaerobic oxidation of methane: Mechanisms, bioenergetics, and the ecology of associated microorganisms. Environ Sci Technol, 42: 6791-6799
Chen J, Jiang X W, Gu J D. 2015. Existence of novel phylotypes of nitritedependent anaerobic methane-oxidizing bacteria in surface and subsurface sediments of the South China Sea. Geomicrobiol J, 32: 1-10
Chen Y, Feng X, He Y, Wang F. 2016. Genome analysis of a Limnobacter sp. identified in an anaerobic methane-consuming cell consortium. Front Mar Sci, 3: 257
Chen Y, Li Y L, Zhou G T, Li H, Lin Y T, Xiao X, Wang F P. 2015. Biomineralization mediated by anaerobic methane-consuming cell consortia. Sci Rep, 4: 5696
Childress J J, Fisher C R, Brooks J M, Kennicutt M C, Bidigare R, Anderson A E. 1986. A methanotrophic marine molluscan (bivalvia, my- tilidae) symbiosis: Mussels fueled by gas. Science, 233: 1306-1308
Cicerone R J, Oremland R S. 1988. Biogeochemical aspects of atmospheric methane. Glob Biogeochem Cycle, 2: 299-327
Conrad R. 2009. The global methane cycle: Recent advances in understanding the microbial processes involved. Environ Microbiol Rep, 1: 285-292
D'Hondt S, Rutherford S, Spivack A J. 2002. Metabolic activity of subsurface life in deep-sea sediments. Science, 295: 2067-2070
Dalton H. 1992. Methane oxidation by methanotrophs. In: Murrell J C, Dalton H, eds. Methane and Methanol Utilizers. Biotechnology Handbooks, vol 5. Boston: Springer. 85-114
Deutzmann J S, Stief P, Brandes J, Schink B. 2014. Anaerobic methane oxidation coupled to denitrification is the dominant methane sink in a deep lake. Proc Natl Acad Sci USA, 111: 18273-18278
Dickens G R. 2003. Rethinking the global carbon cycle with a large, dynamic and microbially mediated gas hydrate capacitor. Earth Planet Sci Lett, 213: 169-183
Divins D L. 2003. Total Sediment Thickness of the World's Oceans & Marginal Seas. NOAA National Geophysical Data Center, Boulder
Egert M, Wagner B, Lemke T, Brune A, Friedrich M W. 2003. Microbial community structure in midgut and hindgut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl Environ Microbiol, 69: 6659-6668
Enzmann F, Mayer F, Rother M, Holtmann D. 2018. Methanogens: Biochemical background and biotechnological applications. AMB Expr, 8:
I,https://doi.org/10.1186/s13568-017-0531-x
Ettwig K F, Butler M K, Le Paslier D, Pelletier E, Mangenot S, Kuypers M M M, Schreiber F, Dutilh B E, Zedelius J, de Beer D, Gloerich J, Wessels H J C T, van Alen T, Luesken F, Wu M L, van de Pas- Schoonen K T, Op den Camp H J M, Janssen-Megens E M, Francoijs K
J, Stunnenberg H, Weissenbach J, Jetten M S M, Strous M. 2010. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature, 464: 543-548
Ettwig K F, Shima S, van de Pas-Schoonen K T, Kahnt J, Medema M H, Op den Camp H J M, Jetten M S M, Strous M. 2008. Denitrifying bacteria anaerobically oxidize methane in the absence of Archaea. Environ Microbiol, 10: 3164-3173
Ettwig K F, van Alen T, van de Pas-Schoonen K T, Jetten M S M, Strous M. 2009. Enrichment and molecular detection of denitrifying metha- notrophic bacteria of the NC10 phylum. Appl Environ Microbiol, 75: 3656-3662
Ettwig K F, Zhu B, Speth D, Keltjens J T, Jetten M S M, Kartal B. 2016. Archaea catalyze iron-dependent anaerobic oxidation of methane. Proc Natl Acad Sci USA, 113: 12792-12796
Evans P N, Parks D H, Chadwick G L, Robbins S J, Orphan V J, Golding S D, Tyson G W. 2015. Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science, 350: 43138
Feng D, Chen D, Roberts H H. 2009. Petrographic and geochemical characterization of seep carbonate from Bush Hill (GC 185) gas vent and hydrate site of the Gulf of Mexico. Mar Pet Geol, 26: 1190-1198
Ferry J G. 2010. The chemical biology of methanogenesis. Planet Space Sci, 58: 1775-1783
Ferry J G, Lessner D J. 2008. Methanogenesis in marine sediments. Ann New York Acad Sci, 1125: 147-157
Fischer R, Thauer R K. 1990. Ferredoxin-dependent methane formation from acetate in cell extracts of Methanosarcina barkeri (strain MS). FEBS Lett, 269: 368-372
Fisher C R, Brooks J M, Vodenichar J S, Zande J M, Childress J J, Jr. R A B. 1993. The co-occurrence of methanotrophic and chemoautotrophic sulfur-oxidizing bacterial symbionts in a deep-sea mussel. Mar Ecol, 14: 277-289
Claypool G E, Kvenvolden K A. 1983. Methane and other hydrocarbon gases in marine sediment. Annu Rev Earth Planet Sci, 11: 299-327
Giovannelli D, d'Errico G, Fiorentino F, Fattorini D, Regoli F, Angeletti L, Bakran-Petricioli T, Vetriani C, Yucel M, Taviani M, Manini E. 2016. Diversity and distribution of prokaryotes within a shallow-water pockmark field. Front Microbiol, 7: 941
Gong W, Hao B, Wei Z, Ferguson Jr. D J, Tallant T, Krzycki J A, Chan M
K. 2008. Structure of the a2s2 Ni-dependent CO dehydrogenase component of the Methanosarcina barkeri acetyl-CoA decarbonylase/syn- thase complex. Proc Natl Acad Sci USA, 105: 9558-9563
Graham D W, Chaudhary J A, Hanson R S, Arnold R G. 1993. Factors affecting competition between type I and type II methanotrophs in two- organism, continuous-flow reactors. Microb Ecol, 25: 1-27
Green P N. 1992. Taxonomy of methylotrophic bacteria. Methane and methanol utilizers. Boston: Springer. 23-84
GroBkopf R, Stubner S, Liesack W. 1998. Novel euryarchaeotal lineages detected on rice roots and in the anoxic bulk soil of flooded rice microcosms. Appl Environ Microb, 64: 4983-4989
Hallam S J, Putnam N, Preston C M, Detter J C, Rokhsar D, Richardson P M, DeLong E F. 2004. Reverse methanogenesis: Testing the hypothesis with environmental genomics. Science, 305: 1457-1462
Hanson R S, Hanson T E. 1996. Methanotrophic bacteria. Microbiol Rev, 60: 439-471
Haroon M F, Hu S, Shi Y, Imelfort M, Keller J, Hugenholtz P, Yuan Z, Tyson G W. 2013. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature, 500: 567-570
He X, Sun L, Xie Z, Huang W, Long N, Li Z, Xing G. 2013. Sea ice in the Arctic Ocean: Role of shielding and consumption of methane. Atmos Environ, 67: 8-13
He Y, Li M, Perumal V, Feng X, Fang J, Xie J, Sievert S M, Wang F. 2016. Genomic and enzymatic evidence for acetogenesis among multiple lineages of the archaeal phylum Bathyarchaeota widespread in marine sediments. Nat Microbiol, 1: 16035
Hinrichs K U, Hayes J M, Sylva S P, Brewer P G, DeLong E F. 1999. Methane-consuming archaebacteria in marine sediments. Nature, 398: 802-805
Hoehler T M, Alperin M J, Albert D B, Martens C S. 1994. Field and laboratory studies of methane oxidation in an anoxic marine sediment: Evidence for a methanogen-sulfate reducer consortium. Glob Bio- geochem Cycle, 8: 451-463
Huang L N, Chen Y Q, Zhou H, Luo S, Lan C Y, Qu L H. 2003. Characterization of methanogenic Archaea in the leachate of a closed municipal solid waste landfill. FEMS Microbiol Ecol, 46: 171-177
Inagaki F, Nunoura T, Nakagawa S, Teske A, Lever M, Lauer A, Suzuki M, Takai K, Delwiche M, Colwell F S, Nealson K H, Horikoshi K, D'Hondt S, Jorgensen B B. 2006. Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments on the Pacific Ocean Margin. Proc Natl Acad Sci USA, 103: 2815-2820
Itavaara M, Salavirta H, Marjamaa K, Ruskeeniemi T. 2016. Chapter one- geomicrobiology and metagenomics of terrestrial deep subsurface microbiomes. Adv Appl Microbiol, 94: 1-77, doi: 10.1016/bs. aambs.2015.12.001
James R H, Bousquet P, Bussmann I, Haeckel M, Kipfer R, Leifer I, Niemann H, Ostrovsky I, Piskozub J, Rehder G, Treude T, Vielstadte L, Greinert J. 2016. Effects of climate change on methane emissions from seafloor sediments in the Arctic Ocean: A review. Limnol Oceanogr, 61: S283-S299
Kallmeyer J, Pockalny R, Ram Adhikari R, Smith D C, D'Hondt S. 2012. Global distribution of microbial abundance and biomass in subseafloor sediment. Proc Natl Acad Sci USA, 109: 16213-16216
Kitidis V, Upstill-Goddard R C, Anderson L G. 2010. Methane and nitrous oxide in surface water along the North-West Passage, Arctic Ocean. Mar Chem, 121: 80-86
Knief C. 2015. Diversity and habitat preferences of cultivated and uncultivated aerobic methanotrophic bacteria evaluated based on pmoA as molecular marker. Front Microbiol, 6: 1346
Knittel K, Boetius A. 2009. Anaerobic oxidation of methane: Progress with an unknown process. Annu Rev Microbiol, 63: 311-334
Knittel K, Losekann T, Boetius A, Kort R, Amann R. 2005. Diversity and distribution of methanotrophic archaea at cold seeps. Appl Environ Microbiol, 71: 467-479
Lang K. 2014. Diversity, ultrastructure, and comparative genomics of "Methanoplasmatales", the seventh order of methanogens. Doctoral Dissertation. Marburg: Universitat Marburg
Lee H S, Lee J C, Lee I K, Moon H B, Chang Y S, Jacobs D R, Lee D H. 2011. Associations among organochlorine pesticides, methanobacter- iales, and obesity in Korean women. PLoS One, 6: e27773
Lesniewski R A, Jain S, Anantharaman K, Schloss P D, Dick G J. 2012. The metatranscriptome of a deep-sea hydrothermal plume is dominated by water column methanotrophs and lithotrophs. ISME J, 6: 2257-2268
Lessner D J. 2001. Methanogenesis Biochemistry. Hoboken: John Wiley & Sons
Lever M A. 2012. Acetogenesis in the energy-starved deep biosphere一A paradox? Front Microbiol, 2: 284
Lipscomb J D. 1994. Biochemistry of the soluble methane monooxygenase. Annu Rev Microbiol, 48: 371-399
Liu Y, Whitman W B. 2008. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann New York Acad Sci, 1125: 171-189
Lloyd K G, Schreiber L, Petersen D G, Kjeldsen K U, Lever M A, Steen A D, Stepanauskas R, Richter M, Kleindienst S, Lenk S, Schramm A, Jorgensen B B. 2013. Predominant archaea in marine sediments degrade detrital proteins. Nature, 496: 215-218
Losekann T, Knittel K, Nadalig T, Fuchs B, Niemann H, Boetius A, Amann R. 2007. Diversity and abundance of aerobic and anaerobic methane oxidizers at the Haakon Mosby Mud Volcano, Barents Sea. Appl Environ MicroBiol, 73: 3348-3362
Lyu Z, Lu Y. 2015. Comparative genomics of three Methanocellales strains reveal novel taxonomic and metabolic features. Environ Microbiol Rep, 7: 526-537
Maupin-Furlow J, Ferry J G. 1996. Characterization of the cdhD and cdhE genes encoding subunits of the corrinoid/iron-sulfur enzyme of the CO dehydrogenase complex from Methanosarcina thermophila. J Bacteriol, 178: 340-346
McCalley C K, Woodcroft B J, Hodgkins S B, Wehr R A, Kim E H, Mondav R, Crill P M, Chanton J P, Rich V I, Tyson G W, Saleska S R. 2014. Methane dynamics regulated by microbial community response to permafrost thaw. Nature, 514: 478-481
McGlynn S E, Chadwick G L, Kempes C P, Orphan V J. 2015. Single cell activity reveals direct electron transfer in methanotrophic consortia. Nature, 526: 531-535
McInerney M J, Struchtemeyer C G, Sieber J, Mouttaki H, Stams A J M, Schink B, Rohlin L, Gunsalus R P. 2008. Physiology, ecology, phylogeny, and genomics of microorganisms capable of syntrophic metabolism. Ann New York Acad Sci, 1125: 58-72
Meng J, Xu J, Qin D, He Y, Xiao X, Wang F. 2014. Genetic and functional properties of uncultivated MCG archaea assessed by metagenome and gene expression analyses. ISME J, 8: 650-659
Meulepas R J W, Jagersma C G, Khadem A F, Stams A J M, Lens P N L. 2010. Effect of methanogenic substrates on anaerobic oxidation of methane and sulfate reduction by an anaerobic methanotrophic enrichment. Appl Microbiol Biotechnol, 87: 1499-1506
Meyerdierks A, Kube M, Kostadinov I, Teeling H, Glockner F O, Reinhardt R, Amann R. 2010. Metagenome and mRNA expression analyses of anaerobic methanotrophic archaea of the ANME-1 group. Environ Microbiol, 12: 422-439
Milkov A V. 2004. Global estimates of hydrate-bound gas in marine sediments: How much is really out there? Earth-Sci Rev, 66: 183-197
Milucka J, Ferdelman T G, Polerecky L, Franzke D, Wegener G, Schmid M, Lieberwirth I, Wagner M, Widdel F, Kuypers M M M. 2012. Zero- valent sulphur is a key intermediate in marine methane oxidation. Nature, 491: 541-546
Mitterer R M. 2010. Methanogenesis and sulfate reduction in marine sediments: A new model. Earth Planet Sci Lett, 295: 358-366
Moran J J, Beal E J, Vrentas J M, Orphan V J, Freeman K H, House C H. 2008. Methyl sulfides as intermediates in the anaerobic oxidation of methane. Environ Microbiol, 10: 162-173
Murrell J C. 1994. Molecular genetics of methane oxidation. Biodegradation, 5: 145-159
Muyzer G, Stams A J M. 2008. The ecology and biotechnology of sulphate- reducing bacteria. Nat Rev Microbiol, 6: 441-454
Na H, Lever M A, Kjeldsen K U, Schulz F, Jorgensen B B. 2015. Uncultured Desulfobacteraceae and Crenarchaeotal group C3 incorporate 13C-acetate in coastal marine sediment. Environ Microbiol Rep, 7: 614622
Nakagawa S, Takai K. 2006. 3 the isolation of thermophiles from deep-sea hydrothermal environments. Method Microbiol, 35: 55-91
Nickel J C, di Primio R, Mangelsdorf K, Stoddart D, Kallmeyer J. 2012. Characterization of microbial activity in pockmark fields of the SW- Barents Sea. Mar Geol, 332-334: 152-162
Niemann H, Losekann T, de Beer D, Elvert M, Nadalig T, Knittel K, Amann R, Sauter E J, Schluter M, Klages M, Foucher J P, Boetius A. 2006. Novel microbial communities of the Haakon Mosby mud volcano and their role as a methane sink. Nature, 443: 854-858
Niu M, Fan X, Zhuang G, Liang Q, Wang F. 2017. Methane metabolizing microbial communities in the sediment of Haima cold seep area, northwest slope of South China Sea. Fems Microbiol Ecol, 93: fix101
Ojima D S, Valentine D W, Mosier A R, Parton W J, Schimel D S. 1993. Effect of land use change on methane oxidation in temperate forest and grassland soils. Chemosphere, 26: 675-685
Orcutt B N, Sylvan J B, Knab N J, Edwards K J. 2011. Microbial ecology of the dark ocean above, at, and below the seafloor. Microbiol Mol Biol Rev, 75: 361-422
Ozuolmez D, Na H, Lever M A, Kjeldsen K U, Jorgensen B B, Plugge C M. 2015. Methanogenic archaea and sulfate reducing bacteria co-cul- tured on acetate: Teamwork or coexistence? Front Microbiol, 6: 492
Padilla C C, Bristow L A, Sarode N, Garcia-Robledo E, Gomez Ramirez E, Benson C R, Bourbonnais A, Altabet M A, Girguis P R, Thamdrup B, Stewart F J. 2016. NC10 bacteria in marine oxygen minimum zones. ISME J, 10: 2067-2071
Paul K, Nonoh J O, Mikulski L, Brune A. 2012. "Methanoplasmatales," thermoplasmatales-related archaea in termite guts and other environments, are the seventh order of methanogens. Appl Environ Microbiol, 78: 8245-8253
Pernthaler A, Dekas A E, Titus Brown C, Goffredi S K, Embaye T, Orphan V J. 2008. Diverse syntrophic partnerships from deep-sea methane vents revealed by direct cell capture and metagenomics. Proc Natl Acad Sci USA, 105: 7052-7057
Pinero E, Marquardt M, Hensen C, Haeckel M, Wallmann K. 2013. Estimation of the global inventory of methane hydrates in marine sediments using transfer functions. Biogeosciences, 10: 959-975
Poulsen M, Schwab C, Borg Jensen B, Engberg R M, Spang A, Canibe N, Hojberg O, Milinovich G, Fragner L, Schleper C, Weckwerth W, Lund P, Schramm A, Urich T. 2013. Methylotrophic methanogenic Ther- moplasmata implicated in reduced methane emissions from bovine rumen. Nat Commun, 4: 1428
Raghoebarsing A A, Pol A, van de Pas-Schoonen K T, Smolders A J P, Ettwig K F, Rijpstra W I C, Schouten S, Damste J S S, Op den Camp H J M, Jetten M S M, Strous M. 2006. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature, 440: 918-921
Reeburgh W S. 2007. Oceanic methane biogeochemistry. Chem Rev, 107: 486-513
Reitner J, Peckmann J, Blumenberg M, Michaelis W, Reimer A, Thiel V. 2005. Concretionary methane-seep carbonates and associated microbial communities in Black Sea sediments. Palaeogeogr Palaeoclimatol Pa- laeoecol, 227: 18-30
Romer M, Sahling H, Pape T, Bohrmann G, SpieB V 2012. Quantification of gas bubble emissions from submarine hydrocarbon seeps at the Makran continental margin (offshore Pakistan). J GEOPHYS RES- OCEANS, 117(C10)
Roussel E G, Bonavita M A C, Querellou J, Cragg B A, Webster G, Prieur D, Parkes R J. 2008. Extending the sub-sea-floor biosphere. Science, 320: 1046
Rudels B, Larsson A M, Sehlstedt P I. 1991. Stratification and water mass formation in the Arctic Ocean: Some implications for the nutrient distribution. Polar Res, 10: 19-32
Ruff S E, Biddle J F, Teske A P, Knittel K, Boetius A, Ramette A. 2015. Global dispersion and local diversification of the methane seep microbiome. Proc Natl Acad Sci USA, 112: 4015-4020
Scheller S, Goenrich M, Boecher R, Thauer R K, Jaun B. 2010. The key nickel enzyme of methanogenesis catalyses the anaerobic oxidation of methane. Nature, 465: 606-608
Schrenk M O, Huber J A, Edwards K J. 2010. Microbial provinces in the subseafloor. Annu Rev Mar Sci, 2: 279-304
Schubert C J, Coolen M J L, Neretin L N, Schippers A, Abbas B, Durisch- Kaiser E, Wehrli B, Hopmans E C, Damste J S S, Wakeham S, Kuypers M M M. 2006. Aerobic and anaerobic methanotrophs in the Black Sea water column. Environ Microbiol, 8: 1844-1856
Semiletov I, Savelieva N, Weller G, Pipko I, Pugach S, Gukov A Y, Vasilevskaya L. 2000. The dispersion of Siberian river flows into coastal waters: Meteorological, hydrological and hydrochemical aspects. In: Lewis E L, Jones E P, Lemke P, Prowse T D, Wadhams P, eds. The Freshwater Budget of the Arctic Ocean. Dordrecht: Springer. 323-366 Shakhova N, Semiletov I, Salyuk A, Yusupov V, Kosmach D, Gustafsson O. 2010. Extensive methane venting to the atmosphere from sediments of the East Siberian Arctic Shelf. Science, 327: 1246-1250
Shima S, Krueger M, Weinert T, Demmer U, Kahnt J, Thauer R K, Ermler U. 2012. Structure of a methyl-coenzyme M reductase from Black Sea mats that oxidize methane anaerobically. Nature, 481: 98-101
Shubenkova O V, Likhoshvai A V, Kanapatskii T A, Pimenov N V. 2010. Microbial community of reduced pockmark sediments (Gdansk Deep, Baltic Sea). Microbiology, 79: 799-808
Smith D D S, Dalton H. 1989. Solubilisation of methane monooxygenase from Methylococcus capsulatus (Bath). Eur J Biochem, 182: 667-671
Sohngen N. 1906. Uber bakterien, welche methan als kohlenstoffnahrung und energiequelle gebrauchen. Zentrabl Bakteriol Parasitenk In- fektionskr, 15: 513-517
Sommer S, Pfannkuche O, Linke P, Luff R, Greinert J, Drews M, Gubsch S, Pieper M, Poser M, Viergutz T. 2006. Efficiency of the benthic filter: Biological control of the emission of dissolved methane from sediments containing shallow gas hydrates at Hydrate Ridge. Glob Biogeochem Cycle, 20: GB2019
Sorokin D Y, Makarova K S, Abbas B, Ferrer M, Golyshin P N, Galinski E
A, Ciordia S, Mena M C, Merkel A Y, Wolf Y I, van Loosdrecht M C M, Koonin E V. 2017. Discovery of extremely halophilic, methyl-re- ducing euryarchaea provides insights into the evolutionary origin of methanogenesis. Nat Microbiol, 2: 17081
Takai K, Horikoshi K. 1999. Genetic diversity of archaea in deep-sea hydrothermal vent environments. Genetics, 152: 1285-1297
Takai K, Nakamura K, Toki T, Tsunogai U, Miyazaki M, Miyazaki J, Hirayama H, Nakagawa S, Nunoura T, Horikoshi K. 2008. Cell proliferation at 122 C and isotopically heavy CH4 production by a hy- perthermophilic methanogen under high-pressure cultivation. Proc Natl Acad Sci USA, 105: 10949-10954
Tong H, Feng D, Cheng H, Yang S, Wang H, Min A G, Edwards R L, Chen Z, Chen D. 2013. Authigenic carbonates from seeps on the northern continental slope of the South China Sea: New insights into fluid sources and geochronology. Mar Pet Geol, 43: 260-271
Valentine D L, Blanton D C, Reeburgh W S, Kastner M. 2001. Water column methane oxidation adjacent to an area of active hydrate dissociation, Eel river Basin. Geochim Cosmochim Acta, 65: 2633-2640
Valentine D L, Reeburgh W S. 2000. New perspectives on anaerobic methane oxidation. Environ Microbiol, 2: 477-484
Valentine D L, Reeburgh W S, Blanton D C. 2000. A culture apparatus for maintaining H? at sub-nanomolar concentrations. J Microbiol Methods, 39: 243-251
Valenzuela E I, Prieto-Davo A, Lopez-Lozano N E, Hernandez-Eligio A, Vega-Alvarado L, Juarez K, Garcia-Gonzalez A S, Lopez M G, Cervantes F J. 2017. Anaerobic methane oxidation driven by microbial reduction of natural organic matter in a tropical wetland. Appl Environ Microbiol, 83: e00645-17
Vanwonterghem I, Evans P N, Parks D H, Jensen P D, Woodcroft B J, Hugenholtz P, Tyson G W. 2016. Methylotrophic methanogenesis discovered in the archaeal phylum Verstraetearchaeota. Nat Microbiol, 1: 16170
Wallmann K, Pinero E, Burwicz E, Haeckel M, Hensen C, Dale A, Ruepke
L.2012. The global inventory of methane hydrate in marine sediments: A theoretical approach. Energies, 5: 2449-2498
Wang F P, Zhang Y, Chen Y, He Y, Qi J, Hinrichs K U, Zhang X X, Xiao X, Boon N. 2014. Methanotrophic archaea possessing diverging me- thane-oxidizing and electron-transporting pathways. Isme J, 8: 10691078
Wegener G, Krukenberg V, Riedel D, Tegetmeyer H E, Boetius A. 2015. Intercellular wiring enables electron transfer between methanotrophic archaea and bacteria. Nature, 526: 587-590
Weiland P. 2010. Biogas production: Current state and perspectives. Appl Microbiol Biotechnol, 85: 849-860
Wu M L, de Vries S, van Alen T A, Butler M K, Op den Camp H J M, Keltjens J T, Jetten M S M, Strous M. 2011. Physiological role of the respiratory quinol oxidase in the anaerobic nitrite-reducing methano- troph ‘Candidatus Methylomirabilis oxyfera'. Microbiology, 157: 890- 898
Xiao K Q, Beulig F, Kjeldsen K U, Jorgensen B B, Risgaard-Petersen N. 2017. Concurrent methane production and oxidation in surface sediment from Aarhus Bay, Denmark. Front Microbiol, 8: 1198
Zhang Y, Henriet J P, Bursens J, Boon N. 2010. Stimulation of in vitro anaerobic oxidation of methane rate in a continuous high-pressure bioreactor. Bioresource Tech, 101: 3132-3138
Zhang Y, Maignien L, Zhao X, Wang F, Boon N. 2011. Enrichment of a microbial community performing anaerobic oxidation of methane in a continuous high-pressure bioreactor. BMC Microbiol, 11: 137
Zhuang G. 2014. Methylotrophic methanogenesis and potential methylated substrates in marine sediment. Doctoral Dissertation. Bremen: University of Bremen
Zhuang G C, Heuer V B, Lazar C S, Goldhammer T, Wendt J, Samarkin V A, Elvert M, Teske A P, Joye S B, Hinrichs K U. 2018. Relative importance of methylotrophic methanogenesis in sediments of the Western Mediterranean Sea. Geochim Cosmochim Acta, 224: 171-186
Zhuang G C, Lin Y S, Bowles M W, Heuer V B, Lever M A, Elvert M, Hinrichs K U. 2017. Distribution and isotopic composition of trimethylamine, dimethylsulfide and dimethylsulfoniopropionate in marine sediments. Mar Chem, 196: 35-46
Zhuang G C, Lin Y S, Elvert M, Heuer V B, Hinrichs K U. 2014. Gas chromatographic analysis of methanol and ethanol in marine sediment pore waters: Validation and implementation of three pretreatment techniques. Mar Chem, 160: 82-90
Zhuang G C, Elling F J, Nigro L M, Samarkin V, Joye S B, Teske A, Hinrichs K U. 2016. Multiple evidence for methylotrophic methanogenesis as the dominant methanogenic pathway in hypersaline sediments from the Orca Basin, Gulf of Mexico. Geochim Cosmochim Acta, 187: 1-20
(Responsible editor: Xiaoxue WANG)

