Prochlorococcus viruses—From biodiversity to biogeochemical cycles
Xilin XIAO1, Qinglu ZENG2, Rui ZHANG1[ Corresponding author (email: ruizhang@xmu.edu.cn) t Corresponding author (email: jiao@xmu.edu.cn)] & Nianzhi JIAO1t
1 State Key Laboratory of Marine Environmental Science, College of Ocean & Earth Sciences, Institute of Marine Microbes and Ecospheres,
Xiamen University, Xiamen 361102, China;
2 Department of Ocean Science and Division of Life Science, Hong Kong University of Science and Technology, Clear Water Bay,
Hong Kong 999077, China
Received October 31, 2017; revised February 5, 2018; accepted July 13, 2018; published online September 28, 2018
Abstract
As the dominant primary producer in oligotrophic oceans, the unicellular picocyanobacterium Prochlorococcus is the smallest and most abundant photosynthetic phytoplankton in the world and plays an important role in marine carbon cycling. Cyanophages that infect Prochlorococcus influence the growth, carbon fixation, diversity, evolution, and environmental adaptation of their hosts. Here, we review studies on the isolation, genomics, and phylogenetic diversity of Prochlorococcus viruses and their interactions with Prochlorococcus. We also review the potential effects of Prochlorococcus viruses on biogeochemical cycling in the ocean.
Keywords Prochlorococcus viruses, Diversity, Genomics, Biogeochemical significance
Citation: Xiao X, Zeng Q, Zhang R, Jiao N. 2018. Prochlorococcus viruses一From biodiversity to biogeochemical cycles. Science China Earth Sciences, 61: 1728-1736, https://doi.org/lQ.lQQ7/sll43Q-Q17-9247-4
1. Introduction
Prochlorococcus, a genus of marine cyanobacteria, is the smallest (diameter 0.5-0.7 pm) and most abundant photosynthetic organism on the planet. Prochlorococcus is widely distributed between 40 N and 40 S and dominates in oli- gotrophic oceans, contributing a significant part of marine primary production (Chisholm et al., 1988; Partensky et al., 1999). In some oceanic regions, Prochlorococcus is an important contributor (up to 80%) to biomass and primary production (Vaulot et al., 1990; Goericke and Welschmeyer, 1993; Campbell et al., 1997; Liu et al., 1997; Jiao and Yang, 2002; Gerikas-Ribeiro et al., 2016). According to model results, the annual mean global abundance of Prochlorococcus is 2.9 0.1乂10 cells, and the Prochlorococcus global net primary production is 4 Gt C yr 1, which represents 8.5% of oceanic net primary production. Under future warming of the global ocean surface, the distribution of Prochlorococcus will likely extend to the south, with an increase in abundance that is geographically dependent; the estimated mean future increase in cell abundance is 29% (Flombaum et al., 2013). The study of Prochlorococcus populations, activity and diversity is important for understanding the responses of marine ecosystems and biogeochemical cycles to global change.
Viruses are the most abundant biological entities in the ocean, with abundances up to 4x1030; this value is more than 10 times higher than those of bacteria and archaea (Suttle, 2005, 2007). Viruses are one of the major biological factors affecting Prochlorococcus ecological characteristics. Owing to their lethality to their hosts, viruses play a significant role in marine ecology and biogeochemical cycles. For example, viruses can affect the food web structure (Wilhelm and earth.scichina.com link.springer.com Suttle, 1999; Suttle, 2007), host evolution (Rohwer and Thurber, 2009; Avrani and Lindell, 2015), and biogeochemical cycles (Fuhrman, 1999; Wilhelm and Suttle, 1999; Jover et al., 2014; Zhang et al., 2014).
In this short review paper, we summarize the research achievements in the study of Prochlorococcus virus isolation, genomics, and phylogenetic diversity and then discuss the potential effects of Prochlorococcus viruses on biogeochemical cycling.
2. Diversity and genomics of Prochlorococcus viruses
2.1 Isolation and taxonomy of Prochlorococcus viruses
Since Prochlorococcus is sensitive to temperature, light and heavy metals, isolating their viruses is challenging. The most widely used isolation technique is a plating method based on ultra-low-melting-point agarose (Sullivan et al., 2003), which involves inoculating Pro99 medium (Moore et al.,2007)with log-phase Prochlorococcus, “helper" bacteria EZ55, and filtered seawater. Prochlorococcus grows on the solid medium and forms a green mat; however, Prochlorococcus cells infected by viruses die, which results in plaques on the solid medium. The bacteria EZ55 can help Prochlorococcus growth in liquid or solid media at low cell concentrations and benefit plaque formation (Morris et al.,
2008).
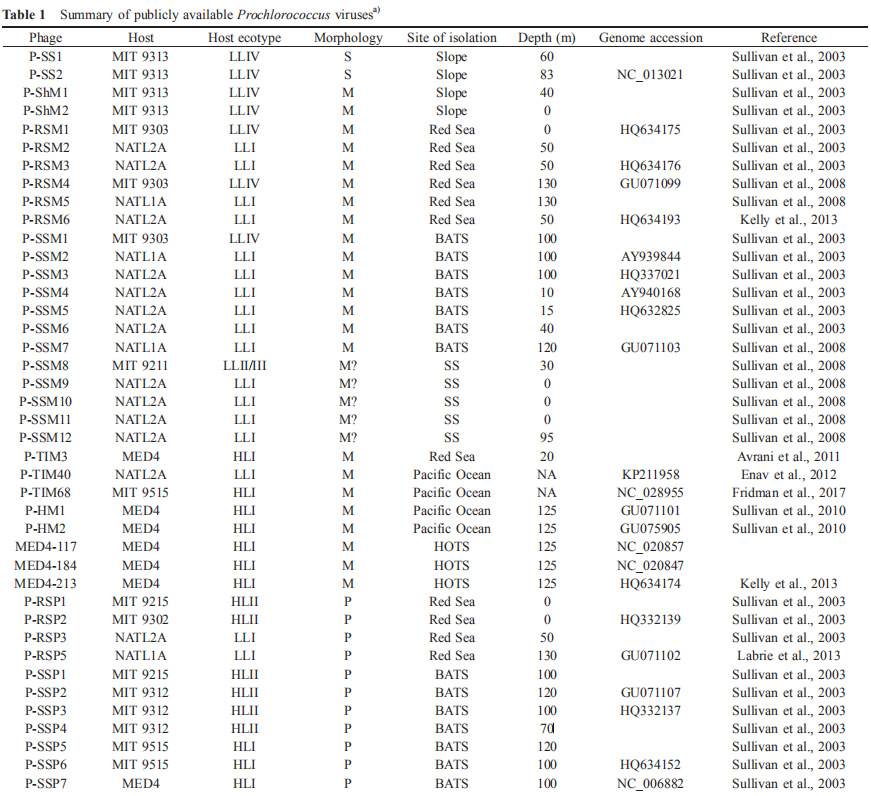
To date, 59 dsDNA Prochlorococcus viruses have been isolated, including 28 myoviruses, 29 podoviruses, and two siphoviruses (Table 1). Similarly, the major morphotypes of viruses infecting Synechocnccus (the sister genus of Prochlorococcus) and Roseobacter (a widely distributed marine heterotrophic bacterial genus) are also myoviruses and po- doviruses (Waterbury and Valois, 1993; Yang et al., 2017). Since a global transmission electron microscopy-based analysis showed that the abundances of these three mor- photypes are comparable, the lack of isolated siphoviruses from Prochlorococcus and other major marine bacterial lineages could be a technique bias.
Based on vertical distribution and light adaptation, Prochlorococcus can be divided into high-light-adapted (HL) and low-light-adapted (LL) ecotypes (Biller et al., 2015). The hosts of the isolated cyanophages described thus far are from the HL ecotypes I and II and the LL ecotypes I, IV and II/III. It is important to note that more than 60% of the reported viruses were isolated from three Prochlorococcus strains, namely, MED4, NATL1A, and NATL2A (Table 1). This confines the study of Prochlorococcus virus diversity, physiology, and ecology and could be the reason that many viral genotypes detected by molecular surveys are not found in isolated cyanophages. Even for Prochlorococcus viruses that have been successfully cultured, there is limited knowledge about virus physiology, such as one-step growth curves and adsorption kinetics, because of the difficulty of obtaining enough material for the quantification of active viral particles. Therefore, further studies isolating and investigating the physiology of viruses infecting Prochlorococcus, especially strains other than MED4, NATL1A, and NATL2A, are urgently needed.
Interestingly, Sullivan et al. (2003) found that 10 of the 18 viruses isolated from S^ynechococcus can infect Prochlorococcus; eight of these viruses infect LL ecotypes, and the remaining two infect both HL and LL ecotypes. However, only three of the 26 viruses isolated from Prochlorococcus can infect Synechococcus. Because of the limited numbers of isolated Prochlorococcus and viruses, it is unknown whether these cross-infection phenomena of Prochlorococcus and Synechococcus viruses are universal. Nevertheless, answering this question is important for our understanding of cyanobacteria-virus interactions. Enav et al. (2012) proposed that cyanophage tRNAs may play a role in the cross-infectivity of oceanic Prochlorococcus and Sy- nechococcus hosts. Doron et al. (2016) suggested that phages with broad host ranges that infect multiple hosts are likely dependent on the effectiveness of host defense strategies rather than on the phage infection process.
2.2 Genome of Prochlorococcus viruses
To date, 16 Prochlorococcus myovirus genomes (Sullivan et al., 2005, 2010; Fridman et al., 2017), 13 podovirus genomes (Sullivan et al., 2005; Labrie et al., 2013) and one siphovirus genome (Sullivan et al., 2009) have been published and show a large variation in genomic content. The podoviruses are similar to phage T7, and the myoviruses are similar to phage T4. The podovirus genomes range from 44.9 to 47.7 kb, with 37-40% G+C contents, and are predicted to contain 50-70 open reading frames (ORFs) (Labrie et al., 2013). The myoviruses have a G+C content ranging from 35% to 38% and genome size ranging from 176.4 to 252.4 kb, and the latter is correlated with the ORF number (221-334) (Sullivan et al., 2010). The only known genome of siphovirus P-SS2 has a size of 108 kb with 132 ORFs and a G+C content of 52.3% (Sullivan et al., 2009). P-SS2 gene homologs are rare in the surface ocean, which suggests that P-SS2-like viruses are mainly distributed in deep photic zones (Sullivan et al.,
2009).
Many host-like genes, such as pigment biosynthesis genes (petE, petF, pebA), photosystem II (PS II) genes (psbA, psbD), photosystem I (PS I) genes (psaJF, psaC, A, B, K, E, D), carbon metabolism-related genes (talC, cp12), phosphate metabolism-related genes (phoH, pstS), nucleotide metabolism-related genes (nrd, mazG) and a vitamin B12 biosynthesis-related gene (cobS), have been found in Prochlorococcus virus genomes (Sullivan et al., 2005;
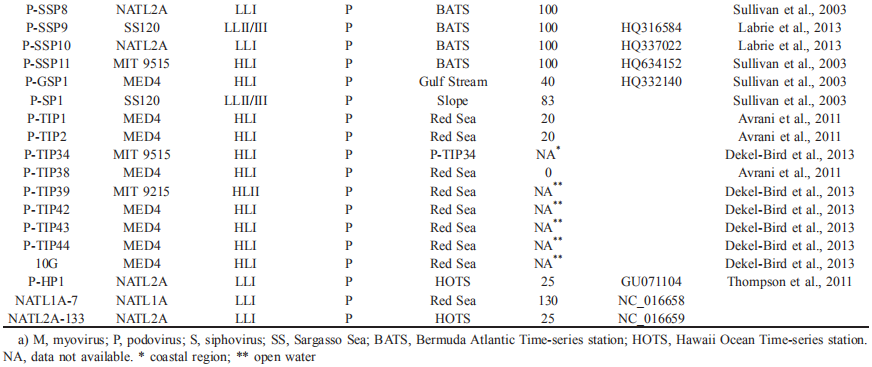
Dammeyer et al., 2008; Fridman et al., 2017). All of these genes, which are known as auxiliary metabolic genes (AMGs), can be expressed in the host cell and are thought to affect host metabolism during infection, providing energy and materials for viral replication (Breitbart et al., 2007; Lindell et al., 2005; Dammeyer et al., 2008; Fridman et al., 2017).
Nearly all the light harvesting-related genes (hoi, pcyA, pebS, cpeT), some electron transfer-related genes (psbD, petE, petF, proX) and carbon, phosphate and nucleotide metabolism-related genes (cp12, phoH, pstS, mazG) appear only in Prochlorococcus myovirus genomes (Sullivan et al., 2005, 2010; Labrie et al., 2013). psbA, hli, nrd and talC are common in the genomes of podoviruses and myoviruses. In addition, myoviruses contain multiple copies of hli, while podoviruses always contain a single copy of hli. One of the possible reasons that the AMGs in myoviruses are more diverse than those in podoviruses is that the former usually have a broader host range and more gene exchange opportunities than the latter.
Studies have shown that some host-derived photosythesis- related genes can be expressed and may play an essential role during infection. Lindell et al. (2005) first reported that Prochlorococcus podovirus P-SSP7 psbA and high-light-inducible (hli) genes were expressed when host gene expression decreased. Evidence from Prochlorococcus myovirus P- TIM68 showed that cyanophage-derived PS II and PS I genes could be transcribed simultaneously. During infection, the host PS I activity was enhanced, while the host PS II activity was not, suggesting that the phage PS I genes replenished host photosythesis and enhanced host PS I activity, thus maintaining the photosynthetic capacity of the host (Fridman et al., 2017). Pigment biosynthesis gene pebS mediated a novel bilin biosynthesis pathway, which is only found in cyanophages and is thought to be more efficient than the host pathway (Dammeyer et al., 2008).
Cyanophages carry the Calvin cycle inhibitor gene cp12 and the pentose phosphate pathway (PPP) transaldolase gene talC (Thompson et al., 2011). It was speculated that, during infection, CP12 proteins inhibit the activity of two key Calvin cycle enzymes, phosphoribulokinase and glycer- aldehyde-3-phosphate dehydrogenase, and shift the carbon flux from the Calvin cycle to PPP. The cyanophage talC sequence is significantly different from the host transaldolase (TalB) but resembles Escherichia coli fructose-6-phos- phate (F6P) aldolase. However, the purified product of talC shows transaldolase activity in vitro, which indicates it might enhance the flux of this key reaction in the host PPP, resulting in the increased production of NADPH and ribose 5- phosphate.
The nucleotide metabolism gene nrd (ribonucleotide reductase gene) has been found in most Prochlorococcus virus genomes. Phage nrd is transcribed with phage DNA replication genes and PPP genes during infection, indicating that nrd may help deoxynucleotide production for virus replication (Lindell et al., 2007; Thompson et al., 2011). In addition, a pyrophosphohydrolase/pyrophosphatase gene (mazG) has been found in most myovirus genomes and is thought to play a role in virus DNA biosynthesis by degrading host DNA and providing more precursors for viral DNA replication.
Prochlorococcus myovirus genomes contain multiple NtcA promoter. NtcA, a 2-oxoglutarate (2OG) dependent nitrogen regulator, which is inactive when 2OG is limited. It was proposed that the cellular nitrogen content of Prochlorococcus decreases after viral infection and likely results in 2OG accumulation, thus leading to the expression of viral NtcA promoter involved nitrogen stress genes (Sullivan et al., 2010). It has been determined that Prochlorococcus virus genomes have phosphate-inducible genes (pstS), which may be part of a possible P-stress response mechanism (Sullivan et al., 2010). Under P-limited conditions, the host P-acqui- sition gene expression is down-regulated in infected cells, while the viral pstS gene expression in infected cells is much higher than that in uninfected cells, which might enhance host P acquisition (Zeng and Chisholm, 2012; Lin et al., 2016).
Cyanophage genome studies have shown that core genes of Prochlorococcus myoviruses contain six known functional genes (psbA, mazG, phoH, hsp20, hli03, cobS), phy- tanoyl-CoA dioxygenase genes, two structural genes, and 16 hypothetical genes (Sullivan et al., 2010). Labrie etal. (2013) showed that Prochlorococcus podovirus genomes contain three variable island regions, and most hypervariable genes are located in the C-terminal regions of the genomes. Me- tagenomic recruitment analysis with 12 cyanopodoviruses showed that podoviruses may represent more than 50% of all recruited reads in samples from the Hawaii Ocean Timeseries Station (HOT) and other marine virome databases. Although high-throughput sequencing and metagenome technology provide more Prochlorococcus virus sequence information, the functions of many viral genes, including AMGs, are still unknown, which suggests more in-house molecular studies are needed.
Interestingly, the Prochlorococcus virus P-SSP7 contains an int gene with conserved amino acid motifs and can possibly integrate its genome into the host genome. Additionally, there is a 42 bp fragment located downstream of the int gene with the same sequence as part of a noncoding strand ofthe leucine tRNA gene in the host genome (Sullivan et al., 2005). This suggests P-SSP7 is possibly a prophage. P- SS2 has also been proposed as a possible prophage (Sullivan et al., 2009). However, no Prochlorococcus prophage has been found thus far. Given that Prochlorococcus lives in oligotrophic environments, it is generally believed that poor nutrient conditions encourage the viruses to choose a lysogenic life style. We thus speculate that a Prochlorococcus prophage may be discovered when more viruses are isolated, which will open a new window to understanding Pro- chlorococcus-virus interactions.
3. Diversity of Prochlorococcus viruses
Viruses lack universally conserved genes, leading to difficulties in investigating viral diversity (Paul et al., 2002). However, some genes are conserved in certain viral groups and can be applied to study their diversity (Rohwer and Edwards, 2002). At present, the gene markers used for diversity studies of Prochlorococcus viruses include the portal protein gene (g20), photosynthetic genes (psbA, psbD, psaA), the major capsid protein gene (MCP gene), and the DNA polymerase gene (DNA pol).
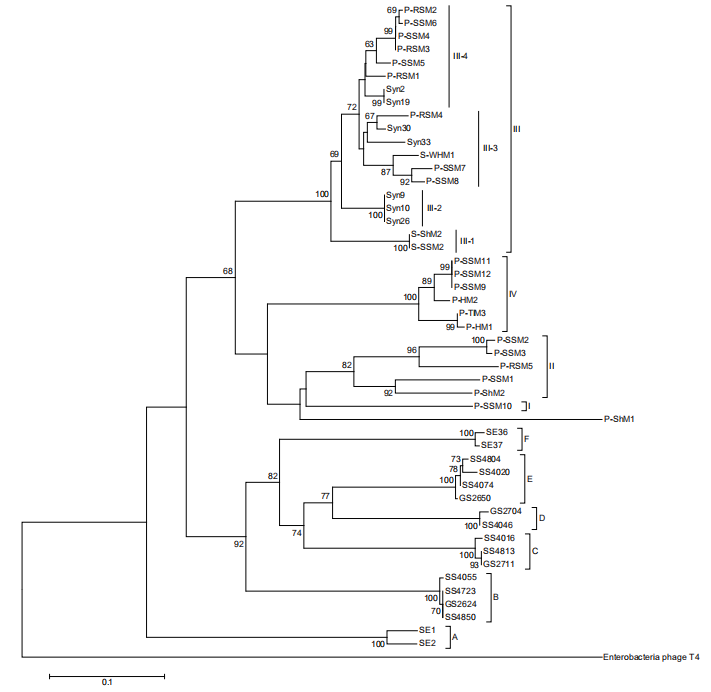
A previous study based on g20 showed cyanomyoviruses can be grouped into four groups (I, II, III, and IV) comprising the isolates and six environmental clades (A-F) with no cultured strains (Sullivan et al., 2008). Group II sequences were found 10 times more often than group I and III sequences in the Global Ocean Survey (GOS) database. We constructed a phylogenetic tree with g20 sequences from 32 published Prochlorococcus myoviruses (Figure 1). When the viral sequence number increased, the four previously established myovirus groups were more clearly differentiated (Figure 1). The six environmental clades still do not have any published isolates, despite being first identified almost 10 years ago. Groups I, II and IV contain myoviruses isolated from Prochlorococcus, while group III includes viruses isolated from Synechococcus with infectivity to Prochlorococcus. In addition, we found Prochlorococcus myoviruses in group III can be further divided into four subgroups (III-1, 2, 3, 4). It would be interesting to investigate whether all clade III myoviruses have the ability to infect Prochlorococcus and Synechococcus.
Based on psbA gene analysis, cyanomyoviruses can be classified into three groups, two isolated from Prochlorococcus and one isolated from Synechococcus (Sullivan et al., 2006). Our analysis of psbA genes within newly ava- liable Prochlorococcus myoviruses demonstrated a similar phylogeny (Figure 2). The cross-infecting myoviruses were located separately in the phylogenetic trees for both structural and functional genes (Figures 1 and 2), suggesting a different evolution pathway than that of the viruses infecting only one cyanobacterial group. The psbD gene analysis results were consistant with the psbA gene analysis (Sullivan et al., 2006). In addition, the g20 gene seemingly showed a better resolution for distinguishing Prochlorococcus myoviruses than psbD and psbA and probably reflects a stronger adaptation of viral portal proteins to host ranges.
According to DNA pol gene analysis, Labrie et al. (2013) classified nine Prochlorococcus podoviruses into two groups (MPP-A, MPP-B) and an outgroup (P-RSP2), which was supported by later studies with more sequences from isolates, PCR amplicons and metagenomics (Dekel-Bird et al., 2013; Huang et al., 2015). The MPP-B group appears to consist of Prochlorococcus and Synechococcus viruses containing psbA, while the MPP-A group does not. Most of the cya- nopodophages are clustered into the MPP-B group, which contains more subgroups than the MPP-A group; this indicates MPP-B podoviruses are more common and have a greater diversity than MPP-A podoviruses in the enviroment (Dekel-Bird et al., 2013). In addition, the PCR-amplified viral psaA gene revealed cyanophages could be classified into six subgroups by their similarity and G+C% content (Hevroni et al., 2015).
In summary, the study of Prochlorococcus virus diversity has mainly focused on isolated viruses, with few PCR amplicon and metagenome data. Our knowledge about the diversity of Prochlorococcus viruses in the global ocean is very limited, considering the wide and dynamic distribution of their hosts. The spatial and temporal dynamics of these viruses remain unknown and require large-scale and longterm ecological investigation. Recently, single cell analysis revealed very high cell-to-cell diversity and significant spatial variation in the Prochlorococcus genome structure, and these characteristics were tightly related to local environmental conditions, such as nutrients, light, and temperature (Kashtan et al., 2014, 2017; Kent et al., 2016). As obligate parasites, viruses have frequent genetic exchanges with their hosts and therefore may have similar genetic diversification. We expect that newly developed techniques with high genetic resolution, such as microfluidic digital PCR, single virus sorting and genomics, and viral tagging (Allen et al., 2011; Brum and Sullivan, 2015), will help us to elucidate the fine-scale diversity of Prochlorococcus viruses.
4. The potential biogeochemical role of Prochlorococcus viruses
Viruses play an important role in the marine biogeochemical cycle by affecting their host's physiology and ecology. As a key player in the oligotrophic ocean, Prochlorococcus contributes an estimated significant part (30-60%) of the total chlorophyll-a in the subtropical surface ocean (Partensky and Garczarek, 2010). The fate ofthis high amount of carbon fixed by Prochlorococcus has a significant biogeochemical impact on global carbon cycling. Generally, the Prochlorococcus mortalities induced by viral lysis and grazing are similar at low and middle latitudes in the North Atlantic Ocean, with an average rate of 0.14 d 1 (range 0.02-0.57 d 1) (Mojica et al., 2016). One study from the deep chlorophyll maximum layer in the subtropical Atlantic Ocean showed the Figure 1 Prochlorococcus myophage neighbor-joining phylogenetic tree based on g20 amino acid sequences. The g20 sequence from the phage T4 was used as an outgroup to root this tree. Support values shown at the nodes are neighbor-joining bootstrap values (only values >60 are shown). Group names follow Sullivan et al. (2008). Scale bar, 0.1 substitutions per amino acid position.
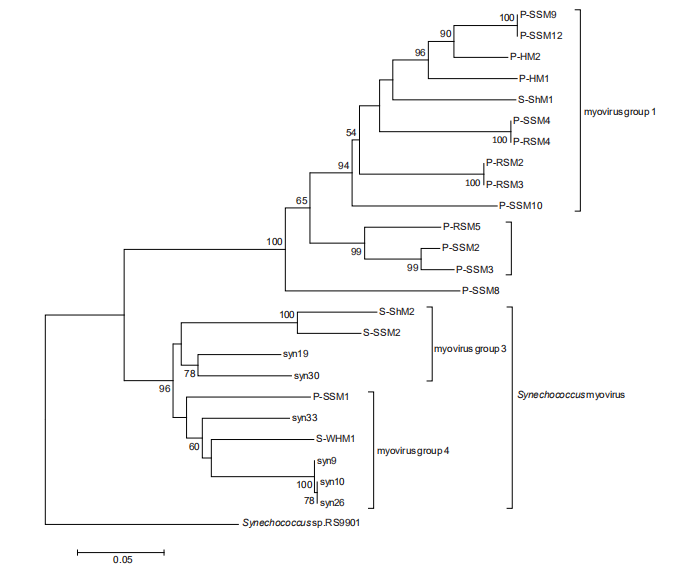
Figure 2 Maximum likelihood phylogenetic analysis of psbA gene sequences from Prochlorococcus myophages. The Synechococcus RS9901 psbA gene sequence was used as an outgroup to root the tree. Bootstrap percentages (values >50) are shown at the nodes. Scale bar, 0.1 substitutions per nucleotide position.
virus-induced Prochlorococcus mortality rate was 0.02 0.03 d 1, accounting for a removal of 3% of the total biomass (Baudoux et al., 2007). In the southern California Current Ecosystem, virus-mediated Prochlorococcus mortality rates are up to 0.25 d-1 (Pasulka et al., 2015). The viral lysis of Prochlorococcus was shown to be related to the growth rate of hosts (Baudoux et al., 2007; Pasulka et al., 2015). In the North Atlantic Ocean, a pattern of reduced viral lysis rates with latitude and a positive relationship between temperature and the viral lysis rate were observed (Mojica et al., 2016), which suggested that the contribution of virus- mediated cell loss to Prochlorococcus mortality is likely to increase in the future. According to these limited field data, it was estimated that virus-mediated mortality is responsible for 1% to 60% of Prochlorococcus cell loss, with large regional variation. In addition, it should be noted that all ofthe published virus-mediated Prochlorococcus mortality rates have been measured by dilution experiments, which were originally developed to estimate microzooplankton grazing on phytoplankton. The application ofthe dilution method for studying the viral lysis of Prochlorococcus is still questionable owing to the sensitivity of Prochlorococcus growth to environmental changes during dilution.
Although the virus-induced mortality rate is not high in Prochlorococcus compared with those in other phytoplankton and bacterioplankton, the potential biogeochemical significance of the viral lysis of Prochlorococcus should not be ignored because of the wide distribution and large population size of Prochlorococcus. Based on an estimated annual abundance of 2.9 0.1乂10 Prochlorococcus cells and celluar carbon quotas ranging from 20 to 60 fg under different nutrient conditions (Bertilsson et al., 2003; Heldal et al., 2003), we estimate 0.6-104.4 Tg C yr-1 is released to the environment by the viral lysis of Prochlorococcus. Dissolved organic matter (DOM) released by lysis contributes significantly to the marine carbon pool and plays a vital role in the marine biogeochemical cycle (Gobler et al., 1997; Middelboe and Jorgensen, 2006). Recently, Zhao et al. (2017) showed that marine cyanobacteria, including Prochlorococcus, release large amounts of fluorescent DOM (FDOM) after viral lysis, which share a similar fluorescence pattern and humic-like composition with deep seawater. This suggests some Prochlorococcus lysates may accumulate in the deep ocean, contributing to the marine carbon pool through the microbial carbon pump (Jiao et al., 2010). In addition, during viral infection, the expression of AMGs may regulate host metabolism and impact the associated biogeochemical cycling. For example, Puxty et al. (2016) showed that viral infections could inhibit 20-50% of cya- nobacterial CO2 fixation during the latent period and that the reduction in CO2 fixation was estimated to be 0.02-5.39 Pg yr-1.
5. Perspective
Prochlorococcus, the smallest free-living autotrophic organism, is the major primary producer in the oligotrophic ocean. Viruses control the abundance, activity, diversity and community structure of Prochlorococcus. However, knowl
edge about Prochlorococcus viruses remains scarce, which limits our understanding of the ecological and biogeochemical roles of Prochlorococcus in the ocean. To address this knowledge gap, we propose several areas for future studies:
(1)Isolation of Prochlorococcus viruses. At present, the isolation of Prochlorococcus viruses is limited to specific hosts (e.g., MED4, NATL1A, and NATL2A) and certain areas (e.g., the Sargasso Sea, the Red Sea), and the isolated viruses and are mainly myoviruses and podoviruses. Metagenome analysis indicates that many types of Prochlorococcus viruses do not have cultured strains. To have a more complete view of the biology and ecology of Prochlorococcus viruses, it is necessary to isolate viruses infecting more Prochlorococcus ecotypes from different ocean regions.
(2)Prochlorococcus-virus interactions. Due to the nature of parasitism, the virus activities mainly rely on host-virus interactions. The investigation of interactions between viruses and Prochlorococcus is of primary importance for understanding the biological and ecological processes involved. However, information about Prochlorococcus-virus interactions is very limited; thus, these interactions should be investigated at genetic, cellular, population and ecosystem levels.
(3)Biogeochemical significance of Prochlorococcus viruses. As an important primary producer, Prochlorococcus contributes significantly to marine carbon cycling. It is unclear how viruses impact the biogeochemical contribution of Prochlorococcus via their interactions at genetic, cellular, population and ecosystem levels. This subject may require the multidisciplinary integration of experimental data, field observations and modeling. In addition, the distribution of Prochlorococcus has been projected to extend to higher latitudes in the warming ocean, and the biogeographic patterns of viruses are also tightly coupled with climate and environmental changes. The simulation and prediction of Prochlorococcus viruses in the future ocean are of great importance for our understanding of the impacts of climate change on marine ecosystems and biogeochemical cycles.
Acknowledgements This work was supported by the Qingdao National Laboratory for Marine Science and Technology (Grant No. QNLM2016ORP0303), and the National Natural Science Foundation of China (Grant Nos. 41522603 & 91428308), and the China National Offshore Oil Corporation (Grant Nos. CNOOC-KJ125FZDXM00TJ001-2014 & CNOOC-KJ125FZDXM00ZJ001-2014).
References
Allen L Z, Ishoey T, Novotny M A, McLean J S, Lasken R S, Williamson S J. 2011. Single virus genomics: A new tool for virus discovery. Plos One, 6: e17722
Avrani S, Wurtzel O, Sharon I, Sorek R, Lindell D. 2011. Genomic island variability facilitates Prochlorococcus-virus coexistence. Nature, 474:
604-608
Avrani S, Lindell D. 2015. Convergent evolution toward an improved growth rate and a reduced resistance range in Prochlorococcus strains resistant to phage. Proc Natl Acad Sci USA, 112: E2191-E2200
Baudoux A C, Veldhuis M J W, Witte H J, Brussaard CP D. 2007. Viruses as mortality agents of picophytoplankton in the deep chlorophyll maximum layer during IRONAGES III. Limnol Oceanogr, 52: 25192529
Bertilsson S, Berglund O, Karl D M, Chisholm S W. 2003. Elemental composition of marine Prochlorococcus and Synechococcus: Implications for the ecological stoichiometry of the sea. Limnol Oceanogr, 48: 1721-1731
Biller S J, Berube P M, Lindell D, Chisholm S W. 2015. Prochlorococcus: The structure and function of collective diversity. Nat Rev Microbiol, 13: 13-27
Breitbart M, Thompson L, Suttle C, Sullivan M. 2007. Exploring the vast diversity of marine viruses. Oceanography, 20: 135-139
Brum J R, Sullivan M B. 2015. Rising to the challenge: Accelerated pace of discovery transforms marine virology. Nat Rev Microbiol, 13: 147-159 Campbell L, Liu H, Nolla H A, Vaulot D. 1997. Annual variability of phytoplankton and bacteria in the subtropical North Pacific Ocean at Station ALOHA during the 1991-1994 ENSO event. Deep-Sea Res Part I-Oceanogr Res Pap, 44: 167-192
Chisholm S W, Olson R J, Zettler E R, Goericke R, Waterbury J B, Welschmeyer N A. 1988. A novel free-living prochlorophyte abundant in the oceanic euphotic zone. Nature, 334: 340-343
Dammeyer T, Bagby S C, Sullivan M B, Chisholm S W, Frankenberg- Dinkel N. 2008. Efficient phage-mediated pigment biosynthesis in oceanic cyanobacteria. Curr Biol, 18: 442-448
Dekel-Bird N P, Avrani S, Sabehi G, Pekarsky I, Marston M F, Kirzner S, Lindell D. 2013. Diversity and evolutionary relationships of T7-like podoviruses infecting marine cyanobacteria. Environ Microbiol, 15: 1476-1491
Doron S, Fedida A, Hernandez-Prieto MA, Sabehi G, Karunker I, Stazic D, Feingersch R, Steglich C, Futschik M, Lindell D, Sorek R. 2016. Transcriptome dynamics of a broad host-range cyanophage and its hosts. ISME J, 10: 1437-1455
Enav H, Beja O, Mandel-Gutfreund Y. 2012. Cyanophage tRNAs may have a role in cross-infectivity of oceanic Prochlorococcus and Sy- nechococcus hosts. ISME J, 6: 619-628
Flombaum P, Gallegos J L, Gordillo R A, Rincon J, Zabala L L, Jiao N Z, Karl D M, Li W K W, Lomas M W, Veneziano D, Vera C S, Vrugt J A, Martiny A C. 2013. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc Natl Acad Sci USA, 110: 9824-9829
Fridman S, Flores-Uribe J, Larom S, Alalouf O, Liran O, Yacoby I, Salama F, Bailleul B, Rappaport F, Ziv T, Sharon I, Cornejo-Castillo F M, Philosof A, Dupont C L, Sanchez P, Acinas S G, Rohwer F L, Lindell D, Beja O. 2017. A myovirus encoding both photosystem I and II proteins enhances cyclic electron flow in infected Prochlorococcus cells. Nat Microbiol, 2: 1350-1357
Fuhrman J A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature, 399: 541-548
Gerikas-Ribeiro C, Dos Santos A L, Marie D, Helena Pellizari V, Pereira Brandini F, Vaulot D. 2016. Pico and nanoplankton abundance and carbon stocks along the Brazilian Bight. Peer J, 4: e2587
Gobler C J, Hutchins D A, Fisher N S, Cosper E M, Sanudo-Wilhelmy S A. 1997. Release and bioavailability of C, N, P Se, and Fe following viral lysis of a marine chrysophyte. Limnol Oceanogr, 42: 1492-1504
Goericke R, Welschmeyer N A. 1993. The marine prochlorophyte Prochlorococcus contributes significantly to phytoplankton biomass and primary production in the Sargasso Sea. Deep-Sea Res Part I-Oceanogr Res Pap, 40: 2283-2294
Heldal M, Scanlan D J, Norland S, Thingstad F, Mann N H. 2003. Elemental composition of single cells of various strains of marine Prochlorococcus and Synechococcus using X-ray microanalysis. Limnol Oceanogr, 48: 1732-1743
Hevroni G, Enav H, Rohwer F, Beja O. 2015. Diversity of viral photo- system-I psaA genes. ISME J, 9: 1892-1898
Huang S, Zhang S, Jiao N, Chen F. 2015. Marine cyanophages demonstrate biogeographic patterns throughout the global ocean. Appl Environ Microbiol, 81: 441X52
Jiao N Z, Yang Y H. 2002. Ecological studies on Prochlorococcus in China seas. Chin Sci Bull, 47: 1243-1250
Jiao N Z, Herndl G J, Hansell D A, Benner R, Kattner G, Wilhelm S W, Kirchman D L, Weinbauer M G, Luo T W, Chen F, Azam F. 2010. Microbial production of recalcitrant dissolved organic matter: Longterm carbon storage in the global ocean. Nat Rev Microbiol, 8: 593-599
Jover L F, Effler T C, Buchan A, Wilhelm S W, Weitz J S. 2014. The elemental composition of virus particles: Implications for marine biogeochemical cycles. Nat Rev Microbiol, 12: 519-528
Kashtan N, Roggensack S E, Rodrigue S, Thompson J W, Biller S J, Coe A, Ding H, Marttinen P, Malmstrom R R, Stocker R, Follows M J, Stepanauskas R, Chisholm S W. 2014. Single-cell genomics reveals hundreds of coexisting subpopulations in wild Prochlorococcus. Science, 344: 416-420
Kashtan N, Roggensack S E, Berta-Thompson J W, Grinberg M, Stepa- nauskas R, Chisholm S W. 2017. Fundamental differences in diversity and genomic population structure between Atlantic and Pacific Prochlorococcus. ISME J, 11: 1997-2011
Kelly L, Ding H, Huang K H, Osburne M S, Chisholm S W. 2013. Genetic diversity in cultured and wild marine cyanomyoviruses reveals phosphorus stress as a strong selective agent. ISME J, 7: 1827-1841
Kent A G, Dupont C L, Yooseph S, Martiny A C. 2016. Global biogeography of Prochlorococcus genome diversity in the surface ocean. ISME J, 10: 1856-1865
Labrie S J, Frois-Moniz K, Osburne M S, Kelly L, Roggensack S E, Sullivan M B, Gearin G, Zeng Q, Fitzgerald M, Henn M R, Chisholm S W. 2013. Genomes of marine cyanopodoviruses reveal multiple origins of diversity. Environ Microbiol, 15: 1356-1376
Lin X Q, Ding H M, Zeng Q L. 2016. Transcriptomic response during phage infection of a marine cyanobacterium under phosphorus-limited conditions. Environ Microbiol, 18: 450-460
Lindell D, Jaffe J D, Johnson Z I, Church G M, Chisholm S W. 2005. Photosynthesis genes in marine viruses yield proteins during host infection. Nature, 438: 86-89
Lindell D, Jaffe J D, Coleman M L, Futschik M E, Axmann I M, Rector T, Kettler G, Sullivan M B, Steen R, Hess W R, Church G M, Chisholm S W. 2007. Genome-wide expression dynamics of a marine virus and host reveal features of co-evolution. Nature, 449: 83-86
Liu H B, Nolla H, Campbell L. 1997. Prochlorococcus growth rate and contribution to primary production in the equatorial and subtropical North Pacific Ocean. Aquat Microb Ecol, 12: 39—47
Middelboe M, Jorgensen NOG. 2006. Viral lysis of bacteria: An important source of dissolved amino acids and cell wall compounds. J Mar Biol Ass, 86: 605-612
Mojica K D A, Huisman J, Wilhelm S W, Brussaard C P D. 2016. Latitudinal variation in virus-induced mortality of phytoplankton across the North Atlantic Ocean. ISME J, 10: 500-513
Moore L R, Coe A, Zinser E R, Saito M A, Sullivan M B, Lindell D, Frois- Moniz K, Waterbury J, Chisholm S W. 2007. Culturing the marine cyanobacterium Prochlorococcus. Limnol Oceanogr Methods, 5: 353362
Morris J J, Kirkegaard R, Szul M J, Johnson Z I, Zinser E R. 2008. Facilitation of robust growth of Prochlorococcus colonies and dilute liquid cultures by "Helper" heterotrophic bacteria. Appl Environ Microbiol, 74: 4530-4534
Partensky F, Hess W R, Vaulot D. 1999. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Mol Biol Rev, 63: 106-127
Partensky F, Garczarek L. 2010. Prochlorococcus: Advantages and Limits
of Minimalism. Annu Rev Mar Sci, 2: 305-331
Pasulka A L, Samo T J, Landry M R. 2015. Grazer and viral impacts on microbial growth and mortality in the southern California Current Ecosystem. J Plankton Res, 37: 320-336
Paul J H, Sullivan M B, Segall A M, Rohwer F. 2002. Marine phage genomics. Comp Biochem Physiol Part B-Biochem Mol Biol, 133: 463-476
Puxty R J, Millard A D, Evans D J, Scanlan D J. 2016. Viruses inhibit CO? fixation in the most abundant phototrophs on Earth. Curr Biol, 26:
1585-1589
Rohwer F, Edwards R. 2002. The phage proteomic tree: A genome-based taxonomy for phage. J Bacteriol, 184: 4529-4535
Rohwer F, Thurber R V. 2009. Viruses manipulate the marine environment. Nature, 459: 207-212
Sullivan M B, Waterbury J B, Chisholm S W. 2003. Erratum: Cyanophages infecting the oceanic cyanobacterium Prochlorococcus. Nature, 424: 1047-1051
Sullivan M B, Coleman M L, Weigele P, Rohwer F, Chisholm S W. 2005. Three Prochlorococcus cyanophage genomes: Signature features and ecological interpretations. PLoS Biol, 3: e144-806
Sullivan M B, Lindell D, Lee J A, Thompson L R, Bielawski J P, Chisholm S W. 2006. Prevalence and evolution of core photosystem II genes in marine cyanobacterial viruses and their hosts. Plos Biol, 4: e234
Sullivan M B, Krastins B, Hughes J L, Kelly L, Chase M, Sarracino D, Chisholm S W. 2009. The genome and structural proteome of an ocean siphovirus: A new window into the cyanobacterial 'mobilome'. Environ Microbiol, 11: 2935-2951
Sullivan M B, Coleman M L, Quinlivan V, Rosenkrantz J E, Defrancesco A S, Tan G, Fu R, Lee J A, Waterbury J B, Bielawski J P, Chisholm S W. 2008. Portal protein diversity and phage ecology. Environ Microbiol, 10: 2810-2823
Sullivan M B, Huang K H, Ignacio-Espinoza J C, Berlin A M, Kelly L, Weigele P R, DeFrancesco A S, Kern S E, Thompson L R, Young S, Yandava C, Fu R, Krastins B, Chase M, Sarracino D, Osburne M S, Henn M R, Chisholm S W. 2010. Genomic analysis of oceanic cya- nobacterial myoviruses compared with T4-like myoviruses from diverse hosts and environments. Environ Microbiol, 12: 3035-3056
Suttle C A. 2005. Viruses in the sea. Nature, 437: 356-361
Suttle C A. 2007. Marine viruses一Major players in the global ecosystem. Nat Rev Microbiol, 5: 801-812
Thompson L R, Zeng Q, Kelly L, Huang K H, Singer A U, Stubbe J, Chisholm S W. 2011. Phage auxiliary metabolic genes and the redirection of cyanobacterial host carbon metabolism. Proc Natl Acad Sci USA, 108: E757-E764
Vaulot D, Partensky FEE, Neveux J, Mantoura RFC, Llewellyn C A. 1990. Winter presence of prochlorophytes in surface waters of the northwestern Mediterranean Sea. Limnol Oceanogr, 35: 1156-1164
Waterbury J B, Valois F W. 1993. Resistance to cooccurring phages enables marine synechococcus communities to coexist with cyanophages abundant in seawater. Appl Environ Microbiol, 59: 3393-3399
Wilhelm S W, Suttle C A. 1999. Viruses and nutrient cycles in the sea. Bioscience, 49: 781-788
Yang Y, Cai L, Ma R, Xu Y, Tong Y, Huang Y, Jiao N, Zhang R. 2017. A novel roseosiphophage isolated from the oligotrophic south china sea. Viruses, 9: 109
Zeng Q, Chisholm S W. 2012. Marine viruses exploit their host's two- component regulatory system in response to resource limitation. Curr Biol, 22: 124-128
Zhang R, Wei W, Cai L L. 2014. The fate and biogeochemical cycling of viral elements. Nat Rev Microbiol, 12: 850-851
Zhao Z, Gonsior M, Luek J, Timko S, Ianiri H, Hertkorn N, Schmitt- Kopplin P, Fang X, Zeng Q, Jiao N, Chen F. 2017. Picocyanobacteria and deep-ocean fluorescent dissolved organic matter share similar optical properties. Nat Commun, 8: 15284
(Responsible editor: Chuanlun ZHANG)

