Evolving paradigms in biological carbon cycling in the ocean
Chuanlun Zhang1,*, Hongyue Dang2, Farooq Azam3, Ronald Benner4, Louis Legendre5, Uta Passow6, Luca Polimene7, Carol Robinson8, Curtis A. Suttle9 and Nianzhi Jiao2,*
ABSTRACT
Carbon is a keystone element in global biogeochemical cycles. It plays a fundamental role in biotic and abiotic processes in the ocean, which intertwine to mediate the chemistry and redox status of carbon in the ocean and the atmosphere. The interactions between abiotic and biogenic carbon (e.g. CO2, CaCOa, organic matter) in the ocean are complex, and there is a half-century-old enigma about the existence of a huge reservoir of recalcitrant dissolved organic carbon (RDOC) that equates to the magnitude of the pool of atmospheric CO2. The concepts of the biological carbon pump (BCP) and the microbial loop (ML) shaped our understanding of the marine carbon cycle. The more recent concept of the microbial carbon pump (MCP), which is closely connected to those of the BCP and the ML, explicitly considers the significance of the ocean's RDOC reservoir and provides a mechanistic framework for the exploration of its formation and persistence. Understanding of the MCP has benefited from advanced 'omics' and novel research in biological oceanography and microbial biogeochemistry. The need to predict the ocean's response to climate change makes an integrative understanding of the BCP, ML and MCP a high priority. In this review, we summarize and discuss progress since the proposal of the MCP in 2010 and formulate research questions for the future.
Keywords: biological carbon pump, microbial loop, microbial carbon pump, ocean carbon cycle, global climate change
INTRODUCTION
The modern oceanaccounts for ~50% of global photosynthesis, with its primary production of organic matter forming the core of the ocean carbon cycle. Thus the ocean has a major influence on the chemistry and redox status of the atmosphere through the net uptake of atmospheric CO2 and net release of molecular oxygen. An early estimate showed that about 2S% of the ocean's primary production was transported to the interior of the ocean (below the euphotic zone) via the biological carbon pump (BCP) [1]; later on, this number was changed to 10-1S% for gravitational sinking with another S% each for passive transport by water motion and active transport by vertical migrators [2]. Carbon transported to the deep ocean ( > 1000 m) is sequestered on timescales of >100years up to 1000years (i.e.the residence time of deep waters). About 0.3% of the ocean's primary production is buried in marine sediments [3,4], some of which eventually forms a major reservoir of organic matter that persists forhundreds of millions of years in rock formations (Fig. 1).
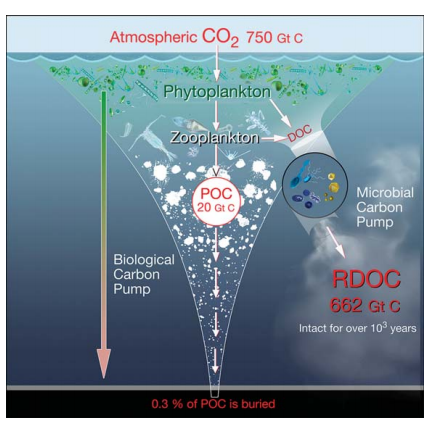
Figure 1. Cycling of biologically produced organic car bon (POC and DOC) in the ocean and links between the seafloorand the atmosphere: the BCP which transports organic matter from the surface to the interior and floor of the ocean; the MCP which converts parts of labile organic carbon into RDOC via microbial activities, mainly by heterotrophic archaea and bacteria, and associated viruses.
Since the industrial revolution, the ocean is estimated to have taken up approximately 2S% of the anthropogenic CO2 [S], resulting in ocean acidification with consequences for biogeochemical and climatological processes and the ocean carbon cycle [6,7,1,8]. Global warming and ocean acidification and their respective consequences influence the functioning of the BCP, a major pathway for sequestering atmospheric CO2 in the ocean. The microbial carbon pump (MCP) [9] provides an additional path for carbon sequestration within the marine ocean carbon cycle [10], which is intimately linked to climate change.
The BCP is the mechanism by which carbon- containing compounds are exported via biological processes from the surface to the deep ocean [11], whereas the MCP addresses the dissolved organic carbon (DOC) pool, specificallythe recalcitrant (R) DOC (Fig. 1), which constitutes the majority of DOC and persists in the ocean for up to 40006000years [12,13]. Hansell [13] defines recalcitrant dissolved organic carbon (RDOC) as ‘DOC that is resistant to rapid microbial degradation and so has accumulated and is observable in the ocean'. Concentrations of DOC in the open ocean range from 360-960 ug/kg (or 30-80 四mol/kg) [14] with significant seasonal variation often seen in surface waters [15]. Accounting for a global ocean inventory of 662 Gt C, the huge DOC pool is almost equal to the carbon dioxide pool (750 Gt C) in the atmosphere. Therefore, the biogeochemical behavior of the DOC pool has important implications for the ocean carbon cycle and climate.
The MCP mediates the transformation of labile carbon to RDOC, which builds on elements of the previously recognized processes involved in ocean carbon cycling and storage [16], namely the BCP, microbial loop (ML) and viral shunt (VS). The functioning of the MCP also impacts nutrient stoichiometry when preferentially remineralizing N and P from dissolved organic matter (DOM). This DOM is produced via the VS [17,18] and other processes such as phytoplankton excretion and zooplankton sloppy feeding [19-24]. This recycling of nutrients enhances local primary production while enriching the remaining DOM in carbon, thus lowering its nutritional value.
The detailed processes of the MCP are currently not well understood. This is largely due to microbial complexity and the vast unresolved chemical structures of DOM compounds. Growing efforts have been devoted to usingmicrobiological and geochemical tools to bridge the gap between microbial omics and organic carbon composition [25-27]. In this review, wediscuss important aspects of the BCP, the ML and the MCP, and summarize progress that has been made concerning the MCP since Jiao et al. [9].
EVOLUTION OF OUR UNDERSTANDING OFTHE MICROBIAL ROLE IN DOC GENERATION AND DEGRADATION
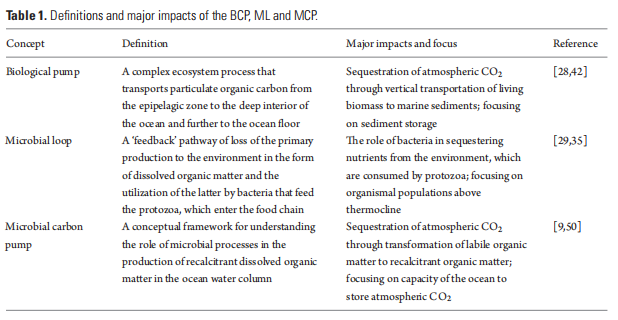
Understandingof the ocean's carboncycle in the late twentieth century was largely promoted bythe BCP (called ‘soft tissue pump' in [28]) and the ML [29]. The term ‘pump' was initially used to refer to the movement of carbon against a concentration gradient between the surface ocean and the deep ocean [28]. Both concepts find their roots in Dugdale and Goering [30], who recognized new (BCP) and regenerated (ML) production in the ocean.
The BCP begins in the euphotic zone where pho- toautotrophic organisms fix dissolved CO2 to produce particulate organic carbon (POC) (Fig. 2). Particulate organic matter (POM) consists of both living and non-living components, and most of it is respired to CO2 by metabolic processes in the epipelagic ecosystem. The subsequent export of a small fraction of the POM is carried out by gravitational flux, vertical migrations of zooplankton and physical subduction of water masses, which remove the organic matter to deeper regions where it accumulates or is respired. The respiratory CO2 at depth is removed from contact with the atmosphere for a period corresponding to the residence time of deep waters, namely tens to hundreds of years below 100 m and thousands of years below 1000 m (Fig. 2). In addition, organic matter in particulate or dissolved form reaching the latter depth via the BCP should be considered as sequestered at the time scale of climate change.
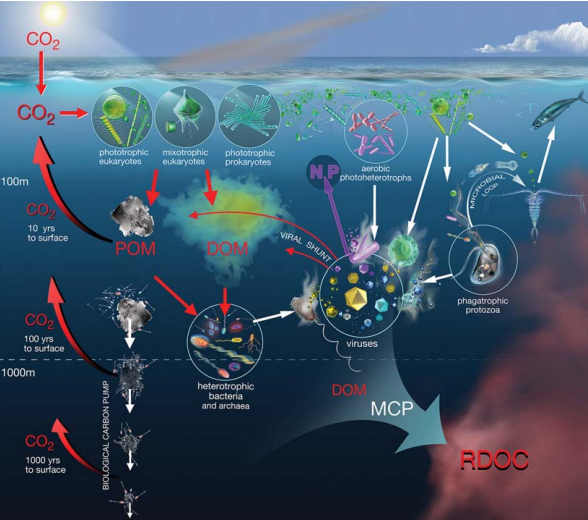
Figure 2. Schematic depiction ofthe BCP the ML andthe MCP. The remineralization length scale in the left part ofthe figure showsthe return of respired CO2 backto the surface, from three depth zones (modified from [31]).
Increasing atmospheric CO2 concentration raises several questions: "(1) Will the ocean continue to take up carbon?(2)At what rate?(3)For how long will the exported carbon remain removed from the atmosphere? These questions address the functioning and efficiency of the future BCP. Global warming and past carbon sequestration (ocean acidification) will also change the BCP leading to the next question: (4) How will the biological pump respond to the consequences of increased carbon input combined with warming? [31]. One scenario suggests that, in the coming decades, decreasing phytoplankton cell size will decrease the downward POC flux from the surface ocean, while changes in zooplankton community structure will decrease the downward POC flux in subsurface waters [32]. However, other predictions suggest alternative outcomes and the answers to these questions are still discussed controversially in the scientific community. A recent report on a transformative understanding of the ocean's BCP to the US National Science Foundation [33] recommended three major research directions addressing "(C) food web regulation of export, (ii) the dissolved-particulate continuum, and (iii) variability of organic transport in space and time. Several large programs, such as the ongoing US-EXPORTS [2] and the UK COMICS [34] programs as well as many other efforts are currently focusing on the BCP.
Though many forms of vertical export can be related to BCP, it mainly focuses on particles that move downward through physical and biological forces (i.e. by gravity and transport by vertically migrating zooplankton). The ML, on the other hand, intimately links intricate interactions between microorganisms and their physical and chemical surroundings [29,35]. The ML focuses on carbon cycling in the water column where bacteria (actually referring to both bacteria and archaea), protozoa and viruses determine the fate of DOM [35]. It was estimated that bacteria could channel up to 50% of marine primary production into the ML, highlighting their importance in the ocean's carbon cycle [35,36]. Similarly, Legendre and Rivkin [37] found euphotic zone, evenwhen most particulate primary production is grazed by metazoans. The ML intertwines with the grazing food web and provides a mechanism to retain nutrients such as N and P in the highly stratified upper oligotrophic oceans by recycling them through pico-phytoplankton, bacteria and microzooplankton [29] (Fig. 2).
The MCP complements and connects the concepts ofBCP andML, additionally includingthe idea of the VS, into a more integrated concept of the cycling ofbiogenic carbon in the ocean. The VS, which refers to the release of carbon and nutrients back into the environment due to cell lysis, is tightly connected to the BCP, the ML and the MCP because cell lysis transforms living POM into DOM and nonliving POM [38,18]. As much as a quarter of the C fixed by phytoplankton is estimated to flow through the VS [18], thereby promoting ecosystem respiration [39]. The released DOM and POM are largely of bacterial origin and, hence, relative to bacterial requirements (because of the carbon required for respiration), have too little carbon relative to other nutrients. This shortage of carbon is exacerbated because of the recalcitrant nature (e.g. cell-wall material) of some of the carbon released by cell lysis. Therefore, as the lysis products are processed by the ML, the more accessible DOM is metabolized, releasing inorganic nutrients, altering pathways of nutrient cycling [40,41] and enriching the pool of less labile DOC. This process directly couples the VS to the ML and MCP, and has been termed the ‘shunt and pump' [17].
The BCP, ML and MCP have distinct ecological or biogeochemical meanings (Table 1) and each has influenced multiple research disciplines (Table 2). These three concepts are fundamental in developing global biogeochemical and ecological models that rely on understanding organismal biology and the interactions between the POC and DOC pools (Fig. 3).
Several reviews provide thorough descriptions of the BCP and the ML (e.g. [36,42,43,31,11]). Here, we focus on recent progress concerning the MCP in the context of the BCP and ML.
PROGRESS ON THE MCP DURING THE LASTEIGHTYEARS
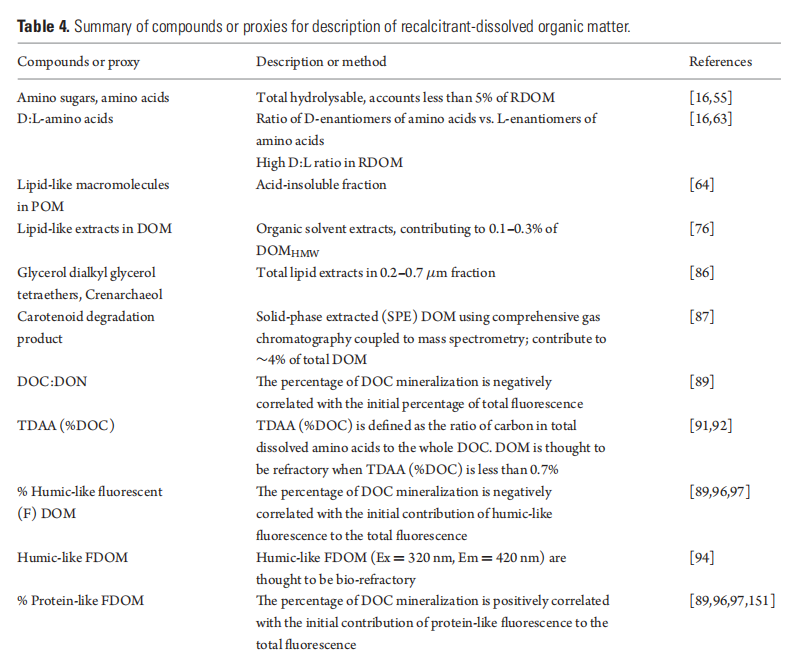
During the last eight years, our understanding of the MCP has advanced appreciably (e.g. [44-49,26,50,51]), specifically addressing some of the questions raised in Jiao et al. [9]. In particular, substantial progress has been made on the composition of recalcitrant DOM, the mechanisms of its formation, the nature of its interactions with ML biogeochemistry and the associated community shifts and trophic dynamics. There were also gains in our understanding of the microbial processing of DOM at various taxonomic and functional group levels (e.g. [52-54]) (Table 3). The state of the art of these topics will be discussed in the remainder of this review.
IDENTIFICATIONAND QUANTIFICATION OFTHE COMPOSITION OF RDOM
According to Hansell et al. [14], less than 1% of the DOC in the ocean is labile and 94% is refractory, while the remaining 5% is classified as semi- labile (note: [13] divided the DOC into labile,semi-labile, semi-refractory, refractory and ultrarefractory). Much of the RDOC production in the ocean can be attributed to microbial activities (e.g. [55]). Kaiser and Benner [56] estimated that 25% of the total organic carbon (including both POC and DOC) was of bacterial origin. Based on the estimates of Hansell et al. [14] and Kaiser and Benner [56], Benner and Herndl [57] calculated that about 10 Pg of semi-labile DOC and 155 Pg of refractory DOC are of bacterial origin. Hansell [13] calculated rates of DOC production for different fractions based on meridional DOC concentration gradients, with the production of RDOC having a rate of 0.043 Pg C/year, which is comparable to the higher end of the RDOC production estimated by Benner and Herndl [57] . Other authors have estimated RDOC production using different criteria. Legendre etal. [50] estimated a rate of 0.2PgC/year for production of RDOC in the world's oceans at all depths using the constraint of RDOC lifetime of >100 years, which is the minimum residence time for the ocean sequestration of carbon in the literature (the origin of the 100-year threshold is explained in [50]). Walker et al. [58] calculated production rates of low-molecular-weight DOM in the range of 0.11-0.14 Pg C/year as a proxy for RDOC production in the deep ocean. These numbers interestingly are comparable to earlier estimates from microbial incubation experiments (0.5-0.6 Pg C/year) [59].
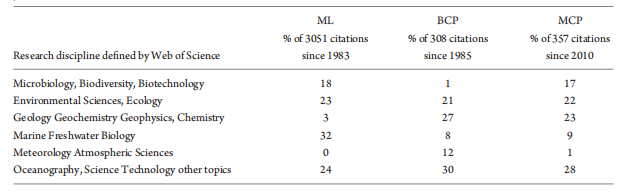
Table 2. Impacts of the three original publications that defined the ML [29], BCP [28] and MCP [9] in different research disciplines, based on the definition of the disciplines in the Web of Science [v.5.29] Core Collection Result Analysis (http://apps.webofknowledge.com). Data were up to 6 July 2018. The percentage value for each discipline is the standardized percentage of the citations in a discipline (minimal 10 citations) vs. the total citations of BCP, ML or MCP since their publication.
Recent efforts to quantify the RDOC pool have been accompanied by progress in the identification of the molecular composition of RDOC and the microbial populations that are responsible for its production in the ocean water column. Microbial RDOC production will be the focus of the following sections, whereas RDOC turnover at deep-sea hydrothermal vents [60-62] and other processes will not be discussed.
Characterization of specific biochemicals in RDOM
Carbohydrates, amino acids and amino sugars Early studies examined the composition of RDOC based on measurements of common biochemicals, such as carbohydrates, amino acids and lipids. Ogawa et al. [55] reported the transformation of labile substrates (D-glucose and D-glutamate) into refractory forms of hydrolysable neutral sugars, amino sugars and amino acids that persisted after 1 year in bioassay experiments. The concentrations of these compounds were later confirmed to be similar to those reported for natural deep ocean waters [63] and represented less than 2% of the total RDOC in low-molecular-weight DOC [16]. In particular, D-enantiomers of amino acids have been observed to contribute to the RDOC pool and are predominantly derived from bacterial sources [56,63] . The ratioof the D-amino acids vs. L-amino acids has been used as a proxy for the degree of recalcitrance, which increases dramatically from bulk POM to the refrac- torylow-molecular-weight DOM [16] (Table 4).
Microbial lipids
Microbial lipids may be important compounds contributing to the RDOC pool in the ocean [64]. Some lipids are much more resistant to degradation than carbohydrates or proteins (hydrolysed to amino acids) [16] and can be preserved in sediments or rocks for hundreds of millions or billions of years [65-67]. Most studies of microbial lipids have been conducted in sediments or POM (e.g. [68-71]) because of the requirement for a large amount of organic material for lipid analysis. Selective accumulation of the refractory lipid-like material in the water column has been demonstrated by the increasing alkanes in the py- rolyzates of sinking POC as depth increased in the Mediterranean Sea [72]. Alkanes from Proterozoic rocks were also identified as biomarkers of heterotrophic bacteria [66]. These biomarkers might have been derived from MCP activity that contributed to the large DOC pool that may have been 100-1000 times greater than in the modern ocean [73-75]. Lipid-like macromolecules in the deep ocean have similar radiocarbon ages also identified as biomarkers of heterotrophic bacteria [66]. These biomarkers might have been derived from MCP activity that contributed to the large DOC pool that may have been 100-1000 times greater than in the modern ocean [73-75]. Lipid-like macromolecules in the deep ocean have similar radiocarbon ages and 513C values as the majority (~70%) of the uncharacterized acid-insoluble fraction, indicating that the bulk POC may be compositionally similar to the lipid-like macromolecules [64] (Table 4).
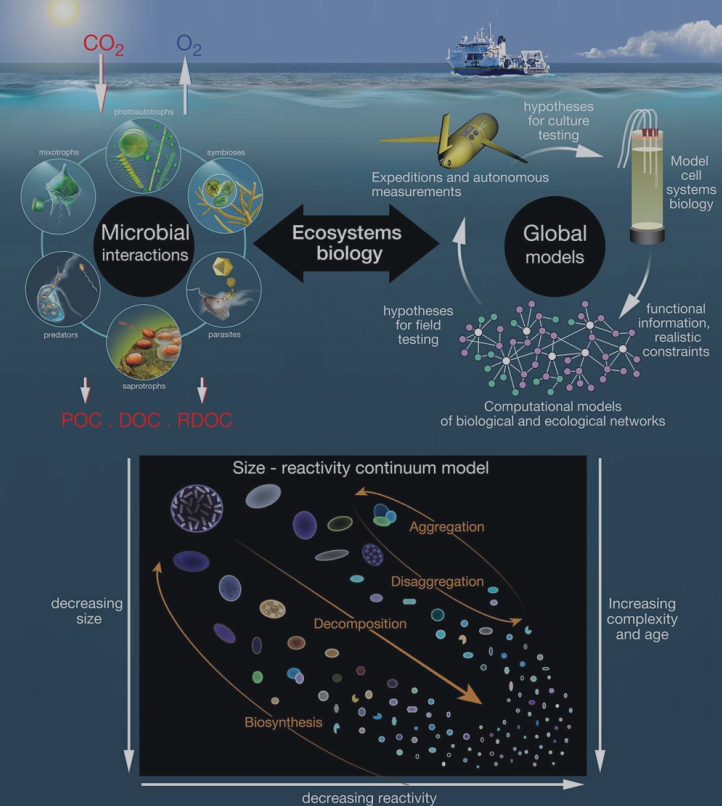
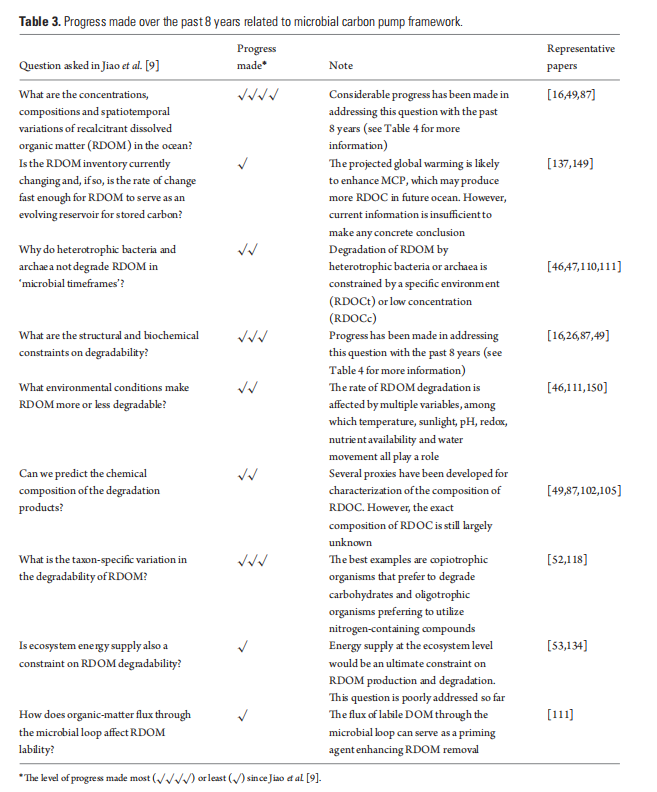
Figure 3. Global biogeochemical and ecological models rely on the present understanding of organismal biology and the interactions between the POM and DOM carbon pools. ModifiedfromWorden etal. [116]. The inset panel isfrom BennerandAmon [16], showingadecreasing sizeand reactivityand an increasing complexity and age of organic molecules along the decomposition pathway. Small dissolved molecules comprise the bulk of RDOC.
The greater ages of lipid-like material than carbohydrate- and protein-like substances were also observed in the DOM pool of the open Atlantic and Pacific Oceans [76]. In particular, the deepwater lipid extract was 13-14 kyr older than the corresponding protein- and carbohydrate-like components in the DOM. This lipid extract was also up to 1 kyr older than the high-molecular-weight DOM. However, the 513C values of the high-molecular- weight DOC were more similar to the carbohydrate- and protein-likesubstances than to the lipidextracts, in contrast to the observations of POC [64]. This suggests that deep-ocean POM and DOM have different origins, with the latterhavingundergone more extensive recycling [76] (Table 4).

Hwang et al. [64] and Loh etal. [76] did not identify specific lipid compositions in either the POM or DOM fractions. However, numerous studies focusing on POM have shown diverse lipid biomarkers from planktonic archaea, bacteria and phytoplankton [77-81,69,82,83]. In particular, crenarchaeol was identified as a major glycerol dialkyl glycerol tetraether (GDGT) biomarker for planktonic Thau- marchaeota that are present in the global ocean at a total inventory of 1028 cells [84]. GDGTs can be preserved in sediments for millions of years [85] and can be a significant component of the lipids in the RDOC pool (Table 4). Because Thaumarchaeota cell size is small, they are more abundant in the DOM fraction (operationally defined as the fraction passing through a ?0.7-^m filter) than the particulate organic fraction [86]. Measurements of the dissolved phases of lipids give total GDGT abundance in the tens of nanograms per liter range [86]; however, once the organisms die, their core lipids may be incorporated into larger particles (0.7- to 60-^m size fraction) that can be more quickly transported into the deeper ocean and buried in marine sediments (Table 4). The same mechanism may apply to bacterial lipid accumulation in the POM fraction that is preserved in marine sediments. It is unknown, however, how much bacterial or archaeal lipids are actually present in the uncharacterized fraction of the RDOM because the uncharacterized RDOM is largely acid-insoluble and cannot be identified by regular gas chromatography or liquid chromatography mass spectrometry.
Carotenoid-degradation products
A recent report by Arakawa et al. [87] identified carotenoid-degradation products (CDP) to be a significant component of the aged DOM using solidphase extraction and comprehensivegas chromatography coupled to mass spectrometry. The CDP are a subset of carboxyl-rich alicyclic molecules (C^AM) and have similar nuclear magnetic resonance spectra as C^AM [88]. However, the cyclic head groups and branched methyl side chains, with conjugated double bonds, are defining features of isoprenoids characteristic of numerous unique carotenoids that can be produced by plankton [87]. The CDP-rich DOM fraction was depleted in radiocarbon (14C age >1500 years), indicating a possible long-term accumulation of CDP in the ocean. This was the first direct confirmation of these terpenoids accumulating in refractory DOM andmay provide a distinct pathway for a single class of biosynthetic precursors to transform to refractory DOM [87] (Table 4). However, this pathway can be either biotic or abiotic and the role that microorganisms play in the transformation of carotenoids to RDOM is unknown.
Characterization of RDOM using proxies
DOC:DON ratio,TDAA(%DOC) and fluorescent DOM
Microorganisms preferentially utilize nitrogencontaining molecules. Thus the ratio DOC:DON could be used to indicate the bioavailability of DOM [89]. Jiao et al. [9] noted that DOC:DON (molar ratio) increased from 10.0 in surface labile DOM to 17.4 in deep-sea refractory DOM [90]. Similarly, DOC-normalized total dissolved amino acid (TDAA (%DOC)) may be an indicator of DOC lability [91,92]. Davis and Benner [91] observed that TDAA (%DOC) decreased from >20% in labile DOM to 0.7% in deep-ocean refractory DOM. Humic-like fluorescent DOM was also thought to be bio-refractory as revealed by its good correlation with apparent oxygen utilization in deep ocean water. This relationship is explained as the production of RDOC from in situ microbial degradation of more labile DOC at the expense of oxygen [93,94]. In addition to fluorescence, absorbance could also be used to infer DOM lability. Specific ultraviolet absorbance has been demonstrated to be a good indicator of aromaticity [95], which negatively correlates with the lability of DOM (or positively correlates with DOM recalcitrance) ([89,96,97]) (Table 4).
Coupling between molecular size and radiocarbon age of DOC
It has been observed that the distribution of total organic carbon in the global ocean is heavily skewed toward the nanometer size range [16]. Ahy- pothesis is that bioavailability of the organic matter decreases with decreasing size and alteration of the organic molecules (Fig. 3 insert), meaning that smaller-sized classes of organic molecules are more slowly remineralized by microorganisms [98,16]. This has been confirmed by approaches coupling the chemical composition and radiocarbon content of marine organic matter in different size fractions ([76,58]. In Loh et al. [76], seawater from different depths of the central North Pacific and the Sargasso Sea region of the North Atlantic showed that the A14C values ranged from -5 to -434%o for high-molecular-weight DOM and from -210 to - 539 for low-molecular-weight DOM, with the latter being older than the former by 1650-1850 kyr. The low-molecular-weight DOM was also the most abundant (77-95%) fraction of total DOM, consistent with the overall dominance of RDOM in the ocean [14]. Walker etal. [58] examinedthe C:N ratio and 14C age of organic matter in different size classes from the coastal, surface and deep waters of the Pacific Ocean. In all three environments, larger particles were characterized by young ages and nitrogen enrichment and smaller molecules by older ages and nitrogen depletion. The size-age-composition relationship was also observed in marine sediments with pore water DOC being dominated by low- molecular-weight DOM [99].
In addition to the relationships between size, age and composition, a recent study observed declining concentrations of high-molecular-weight DOM correlated with increasing apparent oxygen utilization along the shallow overturning circulation cell of the Mediterranean Sea [93]. Decreases in high- mole cular-weight DOM accounted for about 30% of DOM mineralization. The apparent low-molecular- weight DOM experienced little mineralization, indicating microbes primarily utilized high- molecular-weight molecules, whereas the smaller size classes resisted degradation and were the primary source of recalcitrant DOM in the deep ocean [93].
Characterization of RDOM composition using FT-ICR MS
It is well established that RDOM is composed of less than 10% of common biomolecules such as carbohydrates, amino acids or lipids (see discussion above). Proxies such as the DOC:DON ratio, TDAA (%DOC), fluorescent DOM or the sizeage relationship provide insights about the composition and reactivity of DOM, but additional analytical approaches are needed to understand RDOM composition. One approach, Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS), has gained popularity in recent years because it identifies thousands of molecular formulae, which can be further analysed in detail. FT-ICR MS was proposed over 20 years ago [100] and has been increasingly applied in the characterization of changes in DOM composition in both terrestrial and marine environments and along environmental gradients [49,88,101-106].
A number of proxies have been developed based on characterization of DOM using FT-ICR MS. C^AM are commonly believed to be refractory and occur as the most abundant components of DOM in the deep ocean. Using the FT-ICR MS technique, Hertkorn et al. [88] identified over 613 C^AM (Table 4), which can be constrained by the double bond equivalent (DBE) normalized to C (DBE/C = 0.30-0.68), H (DBE/H = 0.200.95) or O (DBE/O = 0.77-1.75) within the van Krevelen diagram. These compounds are characterized by abundant carboxyl groups and alicyclic rings commonly found in terpenoids that occur as membrane constituents or secondary metabolites in diverse prokaryotic and eukaryotic organisms [107]. Such findings can be linked to the GC/GC MS analysis of the CDP that can account for 4% of the RDOM component [87] , which agrees with the estimate that C^AM account for 8% of the DOC [88]. Lechtenfeld et al. [49] further identified 361 most stable molecular formulae, called the ‘island of stability' (IOS) (Table 4) within the CRAM domain [49] in the Atlantic and Southern Ocean waters. These molecules are deemed potential indicators of refractory DOM in the Southern Ocean; however, it is unknown whether the same IOS compounds exist in other oceanic environments.
Another proxy called the degradation index (Ideg) was developed by Flerus et al. [102] to describe the degradation status of marine DOM analysed with FT-ICR MS from solid-phase extraction (SPE) samples (Table 4). Ideg was calculated using 10 mass peak magnitudes that have either significant linear positive or negative correlation with the A14C values of the SPE-DOM. The value of Ideg ranges between 0 and 1 with higher Ideg indicating older age and greater recalcitrance of the DOM. Analysis of seawater at 37O N and 14 W from the eastern Atlantic Ocean showed that Ideg values increased from 0.756 at 400-500 m to 0.808 at 40005000 m, consistent with the notion that DOM from deeper water is more refractory than shallower water. Likewise, the Idex was developed based on the SPE-DOM samples from the Atlantic Ocean, which needs to be verified in other oceanic regions [102].
Lastly, Medeiros et al. [105] identified 184 molecular formulae (Table 4) using FT-ICR MS and used them to indicate riverine inputs in the deep North Atlantic and North Pacific Oceans. These compounds are most enriched in river water and correlated well with known terrigenous tracers in the deep ocean waters, based on which the authors concluded that terrigenous organic matter can be preserved in the deep ocean [105]. This observation is consistent with the deep-ocean distributions of dissolved lignin phenols一biomarkers derived from terrestrial plants [108] .
FT-ICR MS and nuclear magnetic resonance spectroscopy have been used together to trace the source of deep-ocean RDOC from surface primary production. Zhao et al. [109] observed that cultured picocyanobacteria, Synechococcus and Prochlorococcus, released fluorescent DOM that underwent similar photo-degradation behavior when compared with deep-ocean fluorescent DOM (Table 4). Ultrahigh-resolution mass spectrometry and nuclear magnetic resonance spectroscopy revealed abundant nitrogen-containing compounds in Synechococcus DOM, which may originate from degradation products of the fluorescent phycobilin pigments. Their results suggested that picocyanobacteria are likely to be important sources of marine autochthonous fluorescent DOM, which may accumulate in the deep ocean as RDOC [109].
Proxies of RDOM in carbon cycle studies must be used with caution given the current constraints in defining the composition and reactivity of RDOC. Jiao et al. [46] used the term RDOCt to describe RDOC compounds maintaining recalcitrance in a specific environmental context and used RDOCc to describe RDOC compounds being inaccessible to microbes due to their extremely low concentrations. It was debated whether low concentration of any DOC compound is the predominant reason for RDOC to remain recalcitrant in the ocean [47,110]. Recent evidence indicates that only a small fraction of RDOC molecules are too dilute for microbial utilization and that environmental conditions, including exposure to photochemical alterations in surface waters and varying microbial communities, are critical for the removal of RDOC from the ocean [111]. The size-age-composition relationship that organic-matter size is negatively correlated with radiocarbon age and carb on: nitrogen ratios also supports the dominant role of chemical composition (RDOCt) in determining the long persistence of the RDOCpool [58,112].
In addition, if the majority of deep oceanic DOC is RDOCc, namely the dilution hypothesis dominates deep oceanic DOC persistence, the A14C in the deep ocean calculated from a mass balance model of deep oceanic diluted DOC would be difficult to reconcile with the observed A14C(4000- 6000 years) for deep oceanic DOC [113]. This is because, with this observed age constraint, the box model of diluted DOC in the deep ocean would result in either (i) labile DOC comprising a relatively large fraction of bulk DOC but with radiocarbon ages similar to or older than bulk radiocarbon ages or (ii) a smaller labile DOC pool with much younger radiocarbon ages; the latter would be most consistent with a variety of other observations [114].
MECHANISMSAND PROCESSES OF RDOCPRODUCTION
Studies on the MCP have attempted to address the grand challenges of dissecting the composition of the bulk RDOM and identifying the diverse microbial populations responsible for the fate and complexity of RDOM; both are still largely ‘blackboxes'. The research community has reached a consensus that in-depth and integrative characterization of both complex DOM compounds and microbial communities is a prerequisite for exploring the relationship between microbial community composition and the processing of DOM [27,115]. Hopes are high to unveil the intimate linkages between the two black boxes by using the advanced technologies provided by both genomics and bioinformatics, and by mass spectrometry capabilities [25,27,51,116].
Here we present some of the latest advances on focused groups of marine organisms as well as community shifts and trophic dynamics associated with RDOM production.
Carbon metabolism of known organisms
Bacterial metabolism of organic matter is constrained by their physiological capability and biochemical pathways for processing organic molecules. The most studied marine bacteria have been the ‘eutrophic' Roseobacter clade and the ‘oligotrophic' SAR11 clade of marine alphapro- teobacteria [117]; both are numerically dominant and functionally important groups of marine bacteria [52]. These clades have distinct patterns ofDOC utilization, with Roseobacter clade strains mostly taking up carbohydrates and SAR11 preferring nitrogen-containing DOC such as amino acids, which are attributed to different capabilities of ATP binding cassette transporters among these organisms [45,52,118]. Two other studied groups of marine bacteria are the Gammaproteobacteria and the Cytophaga-Flavobacterium-Bacteroides, which are known to be capable of metabolizing macromolecules through the TonB-dependent transporter proteins [52,118]. Cottrel and Kirch- man [43] observed in estuarine and coastal environments that the Cytophaga—Flavobacter cluster showed overrepresentation in the assemblage consuming chitin, N-acetylglucosamine and protein but underrepresentation in the assemblage consuming amino acids. Tang et al. [119] demonstrated through multi-omics analysis and cultivation experiments that the Bacteroidetes strain Gramella flava JLT2011 (Flavobacteria) has the ability to grow on a wide range of polysaccharides such as xylan and homogalacturonan from pectin, which are operated by different polysaccharide utilization loci (PUL) or PUL-like systems. Flavobacteria have also been demonstrated to be a major contributor for the utilization of exopolysaccharides that represent an important source of organic carbon in marine ecosystems [120] . However, Flavobacteria could not completely utilize exopolysaccharides and fluorescent DOM (e.g. humic acid-like substances) produced during metabolism of exopolysaccharides, which may be refractory and may contribute to the carbon storage in the oceans [120]. While these model organisms provide specific knowledge of carbon compounds they metabolize, it is uncertain how these compounds can be identified in natural environments where complex community interactions occur (see below).
Carbon metabolism of natural populations
Studies using individual organisms under laboratory conditions often focus on limited substrates of known compositions. However, the situation is much more complex for natural populations regarding which bacteria may utilize which carbon compounds and whether such compounds in turn may affect specific bacterial community composition [121]. Multiple reports demonstrate that specific carbon compounds can select for particular species or groups of organisms under different environmental conditions [122]. For example, low- molecular-weight molecules (e.g. monomers amino acids, sugars, short chain fatty acids) can be easily transported across cell membranes and may be utilized by most heterotrophic bacteria or archaea. However, it has been demonstrated that different low-molecular-weight organic compounds stimulated growth of different types of bacteria, leading to the suggestion that changing composition of the DOC pool can selectively alter the community structure of bacterioplankton [121]. This is consistent with observations of the distribution of Roseobacter or SAR11 types of organisms selecting for different types of organic substrates (see above). However, it also has been demonstrated that it is the quantity and not the quality of phytoplankton- derived DOC that selects for different types of bacteria in a given range (10-100 〃M) of substrate concentrations [54].
The importance of community composition for the fate of DOM has also been shown [53,115]. For example, in incubation experiments using only < 1.0-^m microbial populations, DOM composition was dominated by compounds with lipid and peptide characteristics; whereas, in incubations with the presence of organisms larger than 1.0 ^m, the DOM compositionfrom the culture experiment was nearly identical to that in the natural water, indicating the role of larger microorganisms in constraining DOM composition in the marine environment [S3]. These studies highlight the importance ofboth microbial community structure and composition or abundance of DOM in the marine system, which should allow distinctionbetween RDOCt and RDOCc to better understand the MCP framework (see above).
The interplay between bacterial community and DOM composition is also examined by comparing particle-attached vs. free-living organisms using genomic tools [123-128]. Despite our awareness of the different ecological strategies of particle- associated and free-living microbes (e.g. [129]), we know little about the principles behind the phylogenetic differences and life strategies between free-living and particle-attached microbes in the marine environment [126,130]. Particle-associated microbes are capable of utilizing a variety of substrates under nutrient-rich conditions. Free-living heterotrophs, on the other hand, often face a massive pool of refractory dissolved organic molecules under oligotrophic conditions [130,131]. However, Zhang et al. [132] observed that the composition of POM was more strongly related to the free-living than to the particle-attached bacterial community, which indicates that POM composition may significantly influence the free-living bacterial community through the release of labile or semi-labile organic matter from particles contributing to the bioavailability of DOC [132]. The nutritional status of the environment may also affect the difference between particle-attached and free-living populations. For example, in the deep ocean when substrates (ammonia, for example) are scarce, particles provide concentrated life-supporting microenvironments. Microorganisms adapted to a particle-attached lifestyle show the dominance of extracellular hydrolytic enzymes; free-living bacteria, on the other hand, are characterized by hydrolytic enzymes typically bound to the cell surface [130]. In the eutrophic surface ocean and estuaries, substrates or nutrients are abundant and organisms were found to be similar between particle -attached and free-living populations [129,133] .
Microbes-DOM interaction at the ecosystem level
The finding ofKujawinski et al. [S3] that incubation experiments using the whole water community resulted in DOM composition similar to the natural water composition highlights the need to examine the microbes-DOM interaction at the ecosystem scale (Fig. 3). This is convincingly demonstrated by a long-term large volume ( > 100 tons) water column (12 m in depth) incubation, which showed solid evidence of the effective microbial transformation of organic matter from labile to refractory states [48]. Another study provides metagenomic evidence of system-level dynamics of microbes-DOM interactions, utilizing the Tara Ocean data that included comprehensive sequences of eukaryotic, prokaryotic and viral lineages from samples collected within the euphotic zone of ocean waters [134]. The increased carbon export in this water column was found to correlate not only with bacteria, particularly Synechococ- cus, but also several unicellular eukaryotic microorganisms including three Rhizaria lineages and three dinoflagellate lineages that have previously not been believed to play important roles for carbon flux. Also important is the finding of a correlation between the abundance of Synechococcus phages and increased carbon export at depth, indicating that phage induced cell lysis promotes particle sinking through enhanced aggregate formation [17], thus increasing carbon export to the deep ocean [134]. The impor- tanceof viruses in deeper water is also highlighted by Zhang et al. [135], who considered viral particles as ‘bottom-up' agents fuelingthe ML in the deep ocean.
Another comprehensive study [136] examined the genomic and transcriptional responses of microbial communities to high-molecular-weight DOM addition in samples from the surface ocean. These authors observed specific resource partitioning of DOM by the bacterial species Idiomarina and Al- teromonas spp. that were most highly represented at the early time points and Methylophaga at the final point of the experiment. Their results demonstrated a temporal succession of taxa, metabolic pathways and chemical transformations associated with high- molecular-weight DOM turnover, suggesting that the cycling of marine DOM may require a coordinated and cooperative effort between different bacterial ‘specialists'.
CASE STUDIES OF INTERACTIONS BETWEEN BCP, MLAND MCP
Case Study 1: MCP dynamics associated with upwelling activities
Jiao et al. [46] hypothesized that microbial activity plays a significant role in mediating the source and/or sink of CO2 in a productive upwelling region. This hypothesis was tested by measuring multiple biogeochemical parameters at two cyclonic- eddy-induced upwelling sites in the western South China Sea, which allowed the formulation of a scenario model of MCP processes under different upwelling conditions.
In the western South China Sea, satellite altimet- ric data identified intensification of two cold-core cyclonic eddies, CE1 (decaying) and CE2 (growing), duringsample collection [46]. In the case of the decaying eddy CE1 (modeling scenario 1, Fig. 4), no phytoplankton bloom occurred and Prochlorococcus dominated. The small-sized non-sinking organic particles favored the transfer of energy and organic matter through the ML pathway rather than through the BCP. The enhanced production of labile organic carbon due to upwelled nutrients and phytoplankton growth stimulated microbial respiration (e.g. net community respiration) and decreased POC flux, which suggested that the MCP is the prevailing mechanism for carbon sequestration. In the case of a growing eddy, CE2 (modeling scenario 2, Fig. 4), the rapid growth ofphytoplankton caused enhancement of POC downward export flux, where the BCP was the prevailing mechanism for carbon sequestration. Further research is needed to validate these models for general applications.
Case Study 2: Modelingthe MCP functions
Lu et al. [137] made an attempt to analyse the MCP-related variables and processes using a coupled physical-ecosystem model that used data collected in the South China Sea and assumed a constant annual production of RDOC of ~0.2 Pg C for global oceans [50]. They also ran the model with different scenarios simulating rising sea-surface temperature and compared the BCP and MCP rates and their relative contributions to carbon sequestration.
The model coupled a physical model from the operational Taiwan Strait N owcast\ Fore cast system [138,139] and a biogeochemistry model based on the Carbon, Silicon, Nitrogen Ecosystem module [140], which was modified to incorporate an explicit RDOC pool and the MCP processes (Fig. 5). With the constraint of a bulk RDOC concentration of 40 ^M [13], and the satellite-based value of primary production, this model estimated the ratio of MCP to BCP (at the depth of 1000 m) to be 1:6.08 in the South China Sea. The annual production rate of RDOC by the MCP averaged over the whole South China Sea domain was estimated to be 1.55 mg C m-2 d-1. The BCP, on the other hand, sequestered 9.43 mg C m-2 d-1.
FUTURE RESEARCH FOCI AND PROSPECTS
Jiao et al. [9] highlighted nine major questions re- gardingMCP processes, which have been addressed at different levels over the past 8 years (Table 3). There is an urgent need to better understand the impacts of global-scale environmental change, including ocean warming and acidification and related deoxygenation and changes in nutrients availability on carbon cycling in the ocean [141]. A central question is how microbial processes contribute to the transformation of organic carbon in the ocean. We advocate three approaches to promote future research in this direction in accordance with Jiao et al. [48].
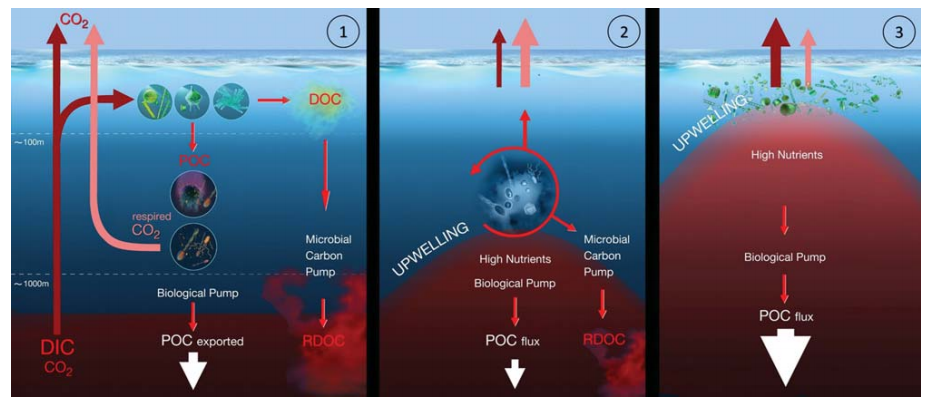
Figure 4. Scenario modelsforthe effects of upwelling on ocean carb on uptake/outgassing dynamics(adopted and modified from [46]). (l)Functioning of the BCP and the MCP in a non-upwelling region of the ocean. (2) Dominance of the MCP in scenario 1 where the total upward CO2 flux exceeds downward POC export flux: nutrients are injected only into the lower layer of the euphotic zone; Prochlorococcus is dominant; microbial respiration is enhanced; CO2 outgassing exceeds POC export; the MCP isthe prevailing mechanismforcarbon sequestration. (3) Dominance of the BCP in scenario 2 where the downward POC flux exceeds the total upward CO2 flux: nutrients are injected intothe upper layer ofthe euphotic zone; diatoms are dominant; POC export exceeds CO2 outgassing; the BCP is the prevailing mechanism for carbon sequestration.
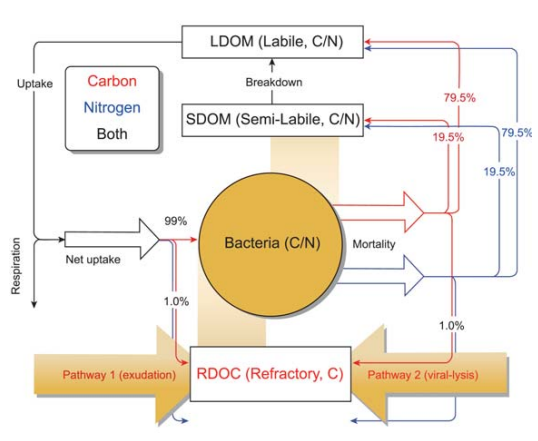
Figure 5. Schematic diagram of the MCP module (from [137]). The RDOC in the model is produced via two bacteria-related pathways: (1) direct exudation by bacteria and (2) passive release from viral lysis of microbial cells. The additional POC degradation pathway [9] is implicitly included by transforming from POC to labile/semi-labile organic carbon and then to RDOC via aforementioned two pathways (see [137] fordetailed explanation).
First, we recommend increased investigation of microbiomes in different natural environments, including a much better coverage of the deep ocean. These studies should integrate various omics approaches (i.e. metagenomics, metatrans crip- tomics, metaproteomics and metabolomics) at all levels of the microbial community (i.e. virus, bacteria, archaea, phytoplankton and zooplankton), as well as at selected time-series locations in the coastal and open ocean to identify how the metabolic capacity of the ocean's microbiome responds to spatial and temporal changes in an environmental context (e.g. [27,133]).
The second proposed approach is to strengthen the understanding of the connections between microbial metabolism and the chemical structure of DOC compounds (e.g. [51]). Bioassays of DOC composition coupled with changes in bacterial communities can now be conducted integrating omics and FT-ICR MS and NMR technologies, which offers the potential for new insights into mechanisms responsible for the formation of RDOCt and RDOCc. In particular, efforts are needed to fully examine the fate of DOM under different trophic conditions and at the ecosystem level [53,134,142].
The third proposed approach is to establish and expand long-term incubation studies employing large-scale facilities, such as the existing Aqua- tron Tower Tank (Dalhousie University, Canada) and the planned Marine Environmental Chamber System (Shandong University, China) under controlled environmental conditions. Using such facilities provides a unique complement to field studies by seeking to mimic ocean-relevant physical, chemical and biological environmental conditions (e.g. vertical stratification) and their variations for long-term experiments. Such experiments are required to provide unique data and insight for testing hypotheses regarding the effects of global environmental change on the ocean carbon cycle [143,144].
We also highlight the need to examine the role of planktonic archaea in the carbon cycle. These archaea, such as Thaumarchaeota, have been recognized to play an important role in the ocean carbon cycle [145].Yet, the claim made 7 years ago that !we are woefully unaware of DOM production (or assimilation) mechanisms in the Archaea' [25] still holds true. The study of archaea is largely hampered by the difficulty of isolating strains from the ocean (e.g. MGII and MGIII). Hence, future efforts should include the development of new technologies for enrichment and isolation of these and other organisms, guided by genomic information [133,146].
The MCP has stimulated provocative and constructive discussions and studies on the processes and mechanisms of RDOC formation and preserva- tion[26,47,111,112,147,148]. Increasing and synergistic efforts will continue to be made to gain further understanding of the ocean carbon cycle through an integration of the concepts of the BCP, ML, VS and MCP, particularly in the context of global ocean circulation (e.g. [111]).
ACKNOWLEDGEMENTS
Writing of this review benefited greatly from discussions by the PICES-ICES WG members and other participants at the MCP Workshop held in Qingdao and the Yanqi Lake Conference on Global Climate Change held in Beijing, both in September 2017. We thank Penghui Li, Yuwu Jiang, Yuan Shen and Nannan Wang for their contribution and constructive comments that improved the quality of the manuscript. The creation of Figures 1-4 was done by Glynn Gorick. This MCP project was supported by the State Key R&D project of China grant Nos. 2018YFA0605802 and 2016YFA0601101 (C.Z.), the National Natural Science Foundation of China (NSFC) grant Nos. 41530105 (C.Z.), 91428308 (C.Z.), 91751207(N.J.), 91428308 (N.J.) and 41676122 (H.D.), the CAS-NSFC project Nos. L1624030 and 2016ZWH008A-008 (N.J.), the Fundamental Research Funds for the Central Universities 20720170107 (NJ.), the Gordon and BettyMoore Foundation award No. 4827 (F.A.), the NSF grant No. OCE-1538602 (U.P.), the UK Natural Environment Research Council (NERC) National Capability on Marine Modelling (L.P.), the UK NERC grant No. NE/R000956/1 (C.R.) and the Leverhulme Trust grant No. RPG-2017-089 (C.R.).
REFERENCES
1.Falkowski PG. The global carbon cycle: a test ofour knowledge of Earth as a system. Science 2000; 290: 291-6.
2.Siegel DA, Buesseler KO and Behrenfeld MJ et al. Prediction of the export and fate of global ocean net primary production: the EXPORTS science plan. Front Mar Sci 2016; 3: 1-10.
3.Dunne JP, Sarmiento JL and Gnanadesikan A. A synthesis of global particle export from the surface ocean and cycling through the ocean interior and on the seafloor. Glob Bio- geochem Cycle 2007; 21: GB4006.
4.Ridgwell A and Arndt S. Why dissolved organics matter: DOC in ancient oceans and past climate change. In: Hansell DA and Carlson CA (eds). BiogeochemistryofMarine Dissolved Organic Matter, 2nd edn. Boston: Academic Press, 2015,1-20.
5.Le Quere C, Andrew RM and Friedlingstein P etal. Global carbon budget2017. Earth Syst SciData 2018; 10: 405-48.
6.Bauer JE, Cai W-J and Raymond PA et al. The changing carbon cycle of the coastal ocean. Nature 2013; 504: 61-70.
7.Cai W-J. Estuarine and coastal ocean carbon paradox: CO2 sinks or sites ofterrestrial carbon incineration? Annu Rev Mar Sci 2011; 3:123-45.
8.Laruelle GG, Cai W-J and Hu X et al. Continental shelves as a variable but increasing global sink for atmospheric carbon dioxide. Nature Comm 2018; 9: 454.
9.Jiao N, Herndl GJ and Hansell DA etal. Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nat Rev Micro 2010; 8: 593-9.
10.Stone R. The invisible hand behind a vast carbon reservoir. Science 2010; 328: 1476-7.
11.Sarmiento JL and Gruber N. Ocean Biogeochemical Dynamics. Princeton: Princeton University Press, 2006, 503.
12.Bauer JE, Williams PM and Druffel ERM. 14C activity of dissolved organic carbon fractions in the north-central Pacific and Sargasso Sea. Nature 1992; 357: 667-70.
13.Hansell DA. Recalcitrant dissolved organic carbon fractions. Annu Rev Mar Sci 2013; 5: 421-45.
14.Hansell DA, Carlson CA and Repeta DJ et al. Dissolved organic matter in the ocean: a controversy stimulates new insights. Oceanog 2009; 22: 202-11.
15.Copin-Montegut G and Avril B. Vertical distribution and temporal variation of dissolved organic carbon in the North-Western Mediterranean Sea. Deep Sea Res Part /1993; 40:1963-72.
16.Benner R and Amon RM.The size-reactivity continuum of major bioelementsintheocean. Annu RevMar Sci 2015; 7:185-205.
17.Suttle CA. Marine viruses一majorplayersinthe global ecosystem. Nat Rev Microbiol 2007; 5: 801-12.
18.Wilhelm SW and Suttle CA. Virusesand nutrient cycles in the sea. Bioscience 1999; 49: 781-8.
19.Arrigo KR. Marine manipulations. Nature 2007; 450: 491-2.
20.Biddanda B and Benner R. Carbon, nitrogen, and carbohydrate fluxes during the production of particulate and dissolved organic matter by marine phytoplankton. LimnolOceanogr 1997; 42: 506-18.
21.Ducklow HW, Carlson CA and Bates NR et al. Dissolved organic carbon as a component of the biological pump in the North Atlantic Ocean. Philos Trans R Soc LondB 1995; 348:161-7.
22.Moller EF. Production of dissolved organic carbon by sloppy feeding in the copepods Acartia tonsa, Centropages typicus, and Temora longicornis. Limnol Oceanogr 2007; 52: 79-84.
23.Roy S, Harris RI and Poulet SA. Inefficient feeding by Calanus helgolandicus and Temora longicornis on Coscinodiscus waile- sii: quantitative estimation using chlorophyll-type pigments and effects on dissolved free amino acids. Mar Ecol Prog Ser 1989; 52: 145-53.
24.Strom S, Benner R and Ziegler S et a/. Planktonic grazers are a potentially important source of marine dissolved organic carbon. L/mno/Oceanogr 1997; 42:1364-74.
25.Kujawinski EB. The impact of microbial metabolism on marine dissolved organic matter. AnnuRevMarSci 2011; 3: 567-99.
26.Lechtenfeld OJ, Hertkorn N and Shen Y eta/. Marine sequestration of carbon in bacterial metabolites. NatCommun2015; 6: 6711.
27.Moran MA, Kujawinski EB and Stubbins A etal. Deciphering ocean carbon in a changing world. Proc Natl Acad Sci USA 2016; 113: 3143-51.
28.Volk T and Hoffert MI. Ocean carbon pumps: analysis of relative strengths and efficiencies in ocean-driven atmospheric CO2 changes. The Carbon Cycle and Atmospheric CO2: Natural Variations Archean to Present. Washington, DC: American Geophysical Union, 1985,99-110.
29.Azam F, Fenchel T and Field JG et al. The ecological role of water-column microbes in the sea. MarEcol Prog Ser 1983; 10: 257-63.
30.Dugdale RC and Goering JJ. Uptake of new and regenerated forms of nitrogen in primary productivity. LimnolOceanogr 1967; 12:196-206.
31.Passow U and Carlson CA. The biological pump in a high CO2 world. MarEcol ProgSer 2012; 470: 249-71.
32.Boyd PW. Toward quantifying the response of the oceans' biological pump to climate change. FrontMar Sci2015; 2:1-15, doi: 10.3389/fmars.2015.00077.
33.Burd A, Buchan A and Church MJ et al. Towards a transformative understanding of the biology of the ocean's biological pump: priorities for future research. Report of the NSF Biology of the Biological Pump Workshop, 1920 February 2016, Hyatt Place New Orleans, New Orleans, LA, 67 pp. doi: 10.1575/1912/8263.
34.Sanders RJ, Henson SA and Martin AP et al. Controls over ocean mesopelagic interior carbon storage (COMICS): fieldwork, synthesis, and modeling efforts. FrontMarSci2016; 3: doi: 10.3389/fmars.2016.00136.
35.Azam F Microbial control of oceanic carbon flux: the plot thickens. Science 1998; 280: 694-6.
36.Fenchel T. The microbial loop—25 years later. JExpMarBiolEcol2008; 366: 99-103.
37.Legendre L and Rivkin R. Planktonic food webs: microbial hub approach. Mar EcolProg Ser2008; 365: 289-309.
38.Suttle CA. Viruses in the sea. Nature 2005: 437, 356-61.
39.Fuhrman JA. Marine viruses and their biogeochemical and ecological effects. Nature 1999; 399: 541-8.
40.Shelford EJ and Suttle CA. Virus-mediated transfer of nitrogen from heterotrophic bacteria to phytoplankton. Biogeosciences 2018; 15:809-19.
41.Weitz JS, Stock CA and Wilhelm SW et al. A multitrophic model to quantify the effects of marine viruses on microbial food webs and ecosystem processes. ISMEJ2015; 9:1352-64.
42.Honjo S, Manganini SJ and Krishfield RA et al. Particulate organic carbon fluxes to the ocean interior and factors controlling the biological pump: a synthesis of global sediment trap programs since 1983. Prog Oceanogr 2008; 76: 217-85.
43.Kirchman DL. Microbial Ecology of the Oceans. New York: Wiley, 2000.
44.Chen JM, Legendre L and Benner R. A recent project shows that the microbial carbon pump is a primary mechanism driving ocean carbon uptake. Natl Sci Rev2018; 5: 458.
45.Jiao N and Zheng Q. The microbial carbon pump: from genes to ecosystems. Appl Environ Microbiol 2011; 77: 7439-44.
46.Jiao N, Zhang Y and Zhou K et al. Revisiting the CO2 ,source' problem in upwelling areas—a comparative study on eddy upwellings in the South China Sea. Biogeosciences 2014; 11: 2465-75.
47.Jiao N, Legendre L and Robinson C etal. Comment on ‘Dilution limitsdissolved organic carbon utilization in the deep ocean'. Science2015; 350:1483.
48.Jiao N, Cai R and Zheng Q et al. Unveiling the enigma of refractory carbon in the ocean. Natl Sci Rev2018; 5: 459-63.
49.Lechtenfeld OJ, Kattner G and Flerus R et al. Molecular transformation and degradation of refractory dissolved organic matter in the Atlantic and Southern Ocean. Geochim Cosmochim Acta 2014; 126: 321-37.
50.Legendre L, Rivkin RB and Weinbauer MG et al. The microbial carbon pump concept: potential biogeochemical significance in the globally changing ocean. Prog Oceanogr 2015; 134: 432-50.
51.Zhang CL. Untangling the role that microbes play in ocean carbon cycle: a new paradigm in marine biogeochemistry. Sci China Earth Sci 2017; 60: 409-12.
52.Dang H and Jiao N. Perspectives on the microbial carbon pump with special reference to microbial respiration and ecosystem efficiency in large estuarine systems. Biogeosciences 2014; 11: 3887-98.
53.Kujawinski EB, Longnecker K and Barott KL et al. Microbial community structure affects marine dissolved organic matter composition. Front Mar Sci 2016; 3: 45.
54.Sarmento H, Morana C and Gasol JM. Bacterioplankton niche partitioning in the use of phytoplankton-derived dissolved organic carbon: quantity is more important than quality. ISMEJ 2016; 10: 2582-92.
55.Ogawa H, Amagai Y and Koike I et al. Production of refractory dissolved organic matter by bacteria. Science 2001; 292: 917-20.
56.Kaiser K and Benner R. Major bacterial contribution to the ocean reservoir of detrital organic carbon and nitrogen. Limnol Oceanogr 2008; 53: 99-112.
57.Benner R and Herndl GJ. Bacterially derived dissolved organic matter in the microbial carbon pump. Microbial Carbon Pump in the Ocean Science 2011: 46-8.
58.Walker BD, Beaupre SR and Guilderson TP etal. Pacific carbon cycling constrained by organic matter size, age and composition relationships. Nature Geosci 2016; 9: 888-91.
59.Brophy JE and Carlson DJ. Production of biologically refractory dissolved organic carbon by natural seawater microbial populations. Deep Sea Research Part A Oceanographic Research Papers 1989; 36: 497-507.
60.Hawkes JA, Rossel PE and Stubbin A et al. Efficient removal of recalcitrant deep-ocean dissolved organic matter during hydrothermal circulation. Nature Geosci 2015; 8: 856-60.
61.Lang SQ, Butterfiekld DA and Lilley MD et al. Dissolved organic carbon in ridge-axis and ridge-flank hydrothermal systems. Geochim Cosmochim Acta 2006; 70: 3830-42.
62.Walter SRS, Jaekel U and Osterholz H et al. Microbial decomposition of marine dissolved organic matter in cool oceanic crust. Nat Geosci 2018; 11: 3349.
63.Kaiser K and Benner R. Biochemical composition and size distribution of organic matter at the Pacific and Atlantic time-series stations. Mar Chem 2009; 113: 63-77.
64.Hwang J and Druffel ER. Lipid-like material as the source of the uncharacterized organic carbon in the ocean? Science2003; 299: 881-4.
65.Brocks JJ, Buick R and Summons RE et al. A reconstruction of Archean biological diversity based on molecular fossils from the 2.78 to 2.45 billion-year-old Mount Bruce Supergroup, Hamersley Basin, Western Australia. Geochim Cos- mochim Acta 2003; 67: 4321-35.
66.Logan GA, Hayes JM and Hieshima GB etal. Terminal Proterozoic reorganization of biogeochemical cycles. Nature 1995; 376: 53-6.
67.Summons RE, Jahnke LL and Hope JM et al. 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature 1999; 400: 554-7.
68.Pancost RD, Damste and S. Carbon isotopic compositions of prokaryotic lipids as tracers of carbon cycling in diverse settings. Chem Geol2003; 195: 29-58.
69.Wakeham SG, Lewis CM and Hopmans EC et al. Archaea mediate anaerobic oxidation of methane in deep euxinic waters of the Black Sea. Geochim CosmochimActa 2003; 67:1359-74.
70.Zhang CL, Li Y and Wall JD et al. Lipid and carbon isotopic evidence of methane-oxidizing and sulfate-reducing bacteria in association with gas hydrates from the Gulfof Mexico. Geol2002; 30: 239-42.
71.Zhang CL, Pancost RD and Sassen R et al. Archaeal lipid biomarkers and isotopic evidence of anaerobic methane oxidation associated with gas hydrates in the Gulfof Mexico. Org Geochem 2003; 34: 827-36.
72.Peulve S, de Leeuw JW and Sicre M-A etal. Characterization of macromolecular organic matter in sediment traps from the northwestern Mediterranean Sea. Geochim Cosmochim Acta 1996; 60: 1239-59.
73.Ridgwell A. Evolution of the ocean's ‘biological pump'. ProcNatlAcadSciUSA 2011; 108:16485-6.
74.Rothman DH, Hayes JM and Summons RE. Dynamics of the neoproterozoic carbon cycle. Proc Natl Acad Sci USA 2003; 100: 8124-9.
75.Tziperman E, Halevy I and Johnston DT et al. Biologically induced initiation of Neoproterozoic snowball-Earth events. Proc NatlAcadSciUSA 2011; 108: 15091-6.
76.Loh AN, Bauer JE and Druffel RM. Variable ageing and storage of dissolved organic components in the open ocean. Nature2004; 430: 877-81.
77.Ingalls AE, Shah SR and Hansman RL etal. Quantifying archaeal community autotrophy in the mesopelagic ocean using natural radiocarbon. Proc Natl Acad Sci USA 2006; 103: 6442-7.
78.Schouten S, Pitcher A and Hopmans EC et al. Intact polar and core glycerol dibiphytanyl glycerol tetraether lipids in the Arabian Sea oxygen minimum zone: I. Selective preservation and degradation in the water column and consequences for the TEX86. Geochim CosmochimActa2012; 98: 228-43.
79.Schubotz F, Wakeham SG and Lipp JS etal. Detection of microbial biomass by intact polar membrane lipid analysis in the water column and surface sediments of the Black Sea. Environ Microbiol2009; 11: 2720-34.
80.Sinninghe Damste JS, Rijpstra WIC and Hopmans EC et al. Distribution of membrane lipids of planktonic Crenarchaeota in the Arabian Sea. ApplEnviron Microbiol 2002; 68: 2997-3002.
81.Turich C, Freeman KH and Bruns MA etal. Lipids of marine Archaea: patterns and provenance in the water-column and sediments. Geochim Cosmochim Acta 2007; 71: 3272-91.
82.Wakeham SG, Amann R and Freeman KH et al. Microbial ecology of the stratified water column of the Black Sea as revealed by a comprehensive biomarker study. Org Geochem 2007; 38: 2070-97.
83.Wei Y, Wang J and Liu J etal. Spatial variations in archaeal lipids of surface water and core-top sediments in the South China Sea and their implications for paleoclimate studies. Appl Environ Microbiol2011; 77: 7479-89.
84.Karner MB, Delong EF and Karl DM. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 2001; 409: 507-10.
85.Kuypers MM, Blokker P and Erbacher J et al. Massive expansion of marine archaea during a mid-Cretaceous oceanic anoxic event. Science 2001; 293: 92-5.
86.Ingalls AE, Huguet C and Truxal LT. Distribution of intact and core membrane lipidsofarchaeal glycerol dialkyl glycerol tetraethers among size-fractionated particulate organic matter in hood canal, puget sound. ApplEnvironMicrobiol 2012; 78: 1480-90.
87.Arakawa N, Aluwihare LI and Simpson AJ et al. Carotenoids are the likely precursor of a significant fraction of marine dissolved organic matter. SciAdv 2017; 3:e1602976.
88.Hertkorn N, Benner R and Frommberger M etal. Characterization of a major refractory component of marine dissolved organic matter. Geochim Cosmochim Acta 2006; 70: 2990-3010.
89.Fellman JB, D'Amore DV and Hood E etal. Fluorescence characteristics and biodegradability of dissolved organic matter in forest and wetland soils from coastal temperate watersheds in southeast Alaska. Biogeochemistry 2008; 88: 169-84.
90.Hopkinson CS and Vallino JJ. Efficient export of carbon to the deep ocean through dissolved organic matter. Nature 2005; 433: 142-5.
91.Davis J and Benner R. Quantitative estimates of labile and semi-labile dissolved organic carbon in the western Arctic Ocean: a molecular approach. Limnol Oceanogr 2007; 52: 2434-44.
92.Shen Y, Chapelle FH and Strom EW et al. Origins and bioavailability of dissolved organic matter in groundwater. Biogeochemistry 2015; 122: 61-78.
93.Martinez-Perez AM, Alvarez-Salgado XA and Aristegui J et al. Deep-ocean dissolved organic matter reactivity along the Mediterranean Sea: does size matter? SciRep2017; 7: 5687.
94.Yamashita Y and Tanoue E. Production of bio-refractory fluorescent dissolved organic matter in the ocean interior. Nature Geosci 2008; 1: 579-82.
95.Weishaar JL, Aiken GR and Bergamaschi BA et al. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol2003; 37: 4702-8.
96.Fellman JB, Hood E and D'Amore DV et al. Seasonal changes in the chemical quality and biodegradability of dissolved organic matter exported from soils to streams in coastal temperate rainforest watersheds. Biogeochemistry 2009; 95: 277-93.
97.Fellman JB, Hood E and Edwards RT et al. Changes in the concentration, biodegradability, and fluorescent properties of dissolved organic matter during stormflows in coastal temperate watersheds. J Geophys Res 2009; 114: G01021,doi: 10.1029/2008JG000790.
98.Amon RMW and Benner R. Bacterial utilization of different size classes of dissolved organic matter. Limnol Oceanogr 1996; 1: 41-51.
99.Burdige DJ and Gardner KG. Molecular weight distribution of dissolved organic carbon in marine sediment pore waters. Mar Chem 1998; 62: 45-64.
100.Kujawinski EB, Hatcher PG and Freitas MA. High resolution Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) of humic and fulvic acids: improvements and comparisons. Anal Chem 2002; 74: 413-9.
101.D'Andrilli J, Cooper WT and Foreman CM et al. An ultrahigh-resolution mass spectrometry index to estimate natural organic matter lability. Rapid Commun Mass Spectrom 2015; 29: 2385-401.
102.Flerus R, Lechtenfeld OJ and Koch BP etal. A molecular perspective on the ageing of marine dissolved organic matter. Biogeosciences 2012; 9: 1935-55.
103.Koch BP, Witt M and Engbrodt R et al. Molecular formulae of marine and terrigenous dissolved organic matter detected by electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Geochim Cosmochim Acta 2005; 69: 3299-308.
104.Kujawinski EB, Longnecker K and Blough NV et al. Identification of possible source markers in marine dissolved organic matter using ultrahigh resolution mass spectrometry. Geochim Cosmochim Acta 2009; 73: 4384-99.
105.Medeiros PM, Seidel M and Gifford SM et al. Microbially-mediated transformations of estuarine dissolved organic matter. Front Mar Sci2017; 4: 69.
106.Sleighter RL and Hatcher PG. Molecular characterization of dissolved organic matter (DOM) along a river to ocean transect of the lower Chesapeake Bay by ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Mar Chem 2008; 110:140-52.
107.Ourisson G, Rohmer M and Poralla K. Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu Rev Microbiol 1987; 41: 301-33.
108.Hernes PJ and Benner R. Terrigenous organic matter sources and reactivity in the North Atlantic Ocean and a comparison to the Arctic and Pacific oceans. MarChem 2006; 100: 66-79.
109.Zhao Z, Gonsior M and Luek J et a/. Picocyanobacteria and deep-ocean fluorescent dissolved organic matter share similar optical properties. Nat Comms 2017; 8:15284.
110.ArrietaJM, Mayol Eand Hansman RL eta/. Response to commenton ‘Dilution limits dissolved organic carbon utilization in the deep ocean'. Science 2015; 350:1483.
111.Shen Y and Benner R. Mixing itup in the ocean carbon cycle and the removal of refractorydissolved organiccarbon. Sci Rep2018; 8: 2542.
112.Amon RMW. Ocean dissolved organics matter. Nature Geosci2016; 9:864-5.
113.Wilson JD and Arndt S. Modeling radiocarbon constraints on the dilution of dissolved organic carbon in the deep ocean. GlobalBiogeochem Cycles2017; 31: 775-86.
114.Hansell DA, Carlson CA and Schlitzer R. Net removal of major marine dissolved organic carbon fractions in the subsurface ocean. Glob Biogeochem Cycles 2012; 26:GB1016.
115.LogueJB,Stedmon CAandKellermanAM etal. Experimental insightsintothe importance of aquatic bacterial community composition to the degradation of dissolved organic matter. ISMEJ2016; 10: 533-45.
116.Worden AZ, Follows MJ and Giovannoni SJ etal. Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes. Science 2015; 347:1257594.
117.Giovannoni SJ. SAR11 bacteria: the most abundant plankton in the oceans. AnnuRevMarSci 2017; 9: 231-55.
118.Tang K, Jiao N and Liu K et al. Distribution and functions of TonB-dependent transporters in marine bacteria and environments: implications for dissolved organic matter utilization. PLoSOne2012; 7: e41204.
119.Tang K, Lin Y and Han Y et al. Characterization of potential polysaccharide utilization systems in the marine Bacteroidetes Gramella flava JLT2011 using a multi-omics approach. Front Microbiol2017; 8: 220.
120.Zhang Z, Chen Y and Wang R et al. The fate of marine bacterial exopolysaccharide in natural marine microbial communities. PLoS One 2015; 10: e0142690.
121.Gomez-Consarnau L, Lindh MV and Gasol JM et al. Structuring of bacterioplankton communities by specific dissolved organic carbon compounds. Envi- ronMicrobiol2012; 14: 2361-78.
122.Rossello-Mora R, Lucio M and Pena A etal. Metabolic evidence for biogeographic isolation of the extremophilic bacterium Salinibacter ruber. ISME J 2008; 2: 242-53.
123.Crump BC, Baross JA and Simenstad CA. Dominance of particle-attached bacteria in the Columbia River estuary, USA. AquatMicrobEcol 1998; 14: 7-18.
124.DeLong EF. Phylogenetic diversity of aggregate-attached vs. free-living marine bacterial assemblages. Limnol Oceanogr 1993; 3: 924-34.
125.Ghiglione JF, Mevel G and Pujo-Pay M etal. Diel and seasonal variations in abundance, activity, and community structure of particle-attached and free- living bacteria in NW Mediterranean Sea. Microb Ecol 2007; 54: 217-31.
126.Moeseneder MM, Winter C and Herndl GJ. Horizontal and vertical complexity of attached and free-living bacteria of the eastern Mediterranean Sea, determined by 16S rDNA and 16S rRNA fingerprints. Limnol Oceanogr 2001; 46: 95-107.
127.Eloe EA, Shulse CN and Fadrosh DW et al. Compositional differences in particle-associated and free-living microbial assemblages from an extreme deep-ocean environment. Environ Microbiol Rep 2011; 3: 449-58.
128.Tarn J, Peoples LM and Hardy K etal. Identification offree-living and particle- associated microbial communities present in hadal regions of the Mariana Trench. Front Microbiol 2016; 7: 665.
129.Dang H and Lovell CR. Microbial surface colonization and biofilm development in marine environments. Microbiol Mol Biol Rev 2016; 80: 91-138.
130.Herndl GJ and Reinthaler T. Microbial control of the dark end of the biological pump. Nature Geosci 2013; 6: 718-24.
131.Lauro FM, Mcdougald D and Thomas T et al. The genomic basis of trophic strategy in marine bacteria. Proc Natl Acad Sci USA 2009; 106: 15527-33.
132.Zhang Y, Xiao W and Jiao N et al. Linking biochemical properties of particles to particle-attached and free-living bacterial community structure along the particle density gradient from freshwater to open ocean. J Geophys Res Biogeosci 2016; 121: 2261-74.
133.Xie W, Luo H and Murugapiran SK et al. Localized high abundance of Marine Group II archaea in the subtropical Pearl River Estuary: implications for their niche adaptation. Environ Microbiol 2018; 20: 734-54.
134.Guidi L, Chaffron S and Bittner L et al. Plankton networks driving carbon export in the oligotrophic ocean. Nature 2016; 532: 465-70.
135.Zhang R, Wei Wand Cai L. The fate and biogeochemical cycling of viral elements. Nat Rev Micro 2014; 12: 850-1.
136.Mccarren J, Becker JW and Repeta DJ et al. Microbial community transcriptomes reveal microbes and metabolic pathways associated with dissolved organic matter turnover in the sea. Proc Natl Acad Sci USA 2010; 107: 16420-7.
137.Lu W, Luo Y and Yan X et al. Modeling the contribution of the microbial carbon pump to carbon sequestration in the South China Sea. Sci China Earth Sci 2018; doi: 10.1007/s11430-017-9180-y.
138.Jiang Y, Chai F and Wan Z et al. Characteristics and mechanisms of the upwelling in the southern Taiwan Strait: a three-dimensional numerical model study. J Oceanogr 2011; 67: 699-708.
139.Lin X, Yan X-H and Jiang Y et al. Performance assessment for an operational ocean model of the Taiwan Strait. Ocean Modell 2016; 102: 27-44.
140.Xiu P and Chai F. Connections between physical, optical and biogeochemical processes in the Pacific Ocean. Prog Oceanogr 2014; 122: 30-53.
141.Jiao N, Liang Y and Zhang Y et al. Comprehensive analysis of carbon pools and fluxes in the China Seas and their adjacent oceans. Sci China Earth Sci 2018; doi: 10.1007/s11430-018-9190-x.
142.Osterholz H, Kirchman DL and Niggemann J et al. Environmental drivers of dissolved organic matter molecular composition in the Delaware Estuary. Front Microbiol 2016; 4: 95.
143.Legendre L, Rivkin RB and Jiao N. Advanced experimental approaches to marine water-column biogeochemical processes. ICES J Mar Sci 2017; 75: 3042.
144.Robinson C, Wallace D and Hyun J-H et al. An implementation strategy to quantify the marine microbial carbon pump and its sensitivity to global change. Natl Sci Rev 2018; 5: 474-80.
145.Dang H and Chen CTA. Ecological energetic perspectives on responses of nitrogen-transforming chemolithoautotrophic microbiota to changes in the marine environment. Front Microbiol 2017; 8: 1246.
146.Zhang CL, Xie W and Martin-Cuadrado AB et al. Marine Group II Archaea, potentially important players in the global ocean carbon cycle. Front Microbiol 2015; 6: 1108.
147.L0nborg C, Alvarez-Salgado XA and Letscher RT et al. Large stimulation of recalcitrant dissolved organic carbon degradation by increasing ocean temperatures. FrontMarSci 2018; 4: 436.
148.Zark M, Christoffers J and Dittmar T. Molecular properties of deep-sea dissolved organic matter are predictable by the central limit theorem: Evidence from tandem FT-ICR-MS. Mar Chem 2017; 191: 9-15.
149.Polimene L, Sailley S and Clark D et al. Biological or microbial carbon pump? The role of phytoplankton stoichiometry in ocean carbon sequestration. J Plankton Res 2017; 39:180-6.
150.Thingstad TF, Hagstrom A and Rassoulzadegan F. Accumulation of degradable DOCinsurface waters: Is itcaused byamalfunctioning microbialloop? Limnol Oceanogr 1997; 42: 398-404.
151.Cory RM and Kaplan LA. Biological lability of streamwater fluorescent dissolved organic matter. Limnol Oceanogr 2012; 57: 1347-60.
152.Walker BD, Primeau FW and Beaupre SR etal. Linked changes in marine dissolved organic carbon molecular size and radiocarbon age. Geophys Res Lett 2016; 43: 10385-93.

